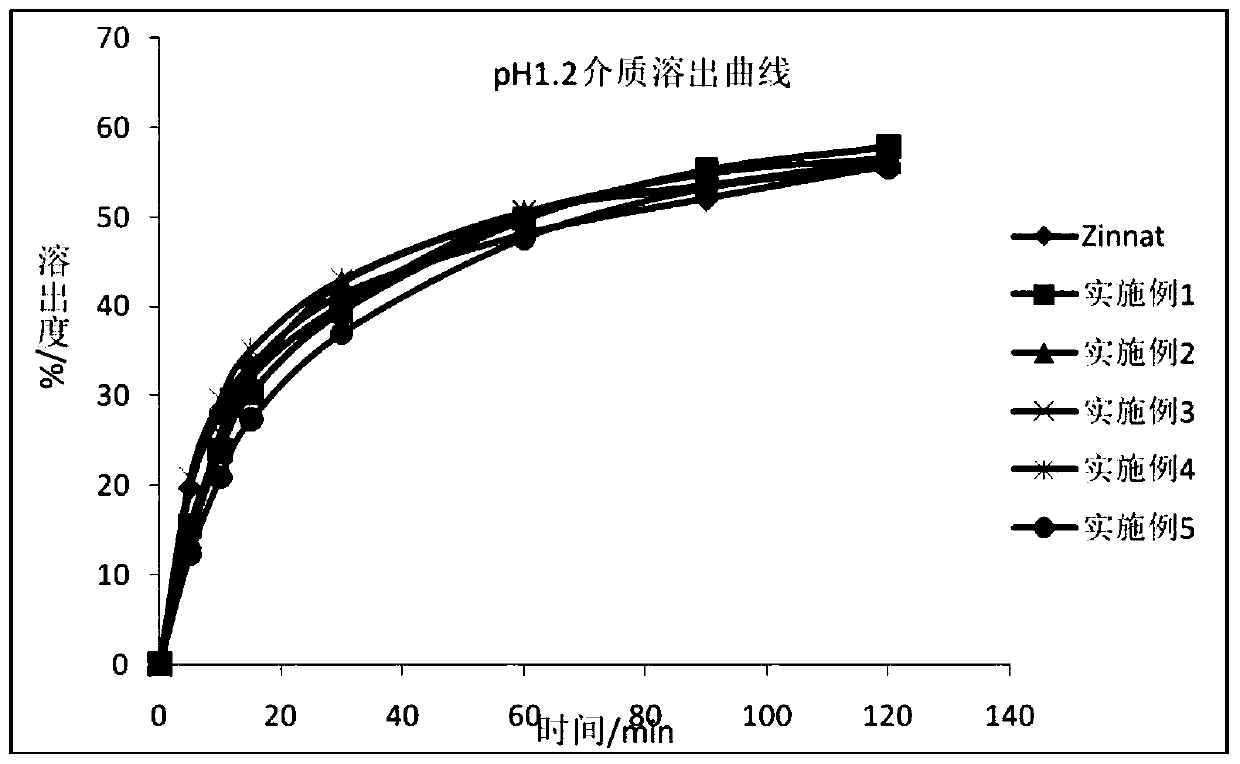
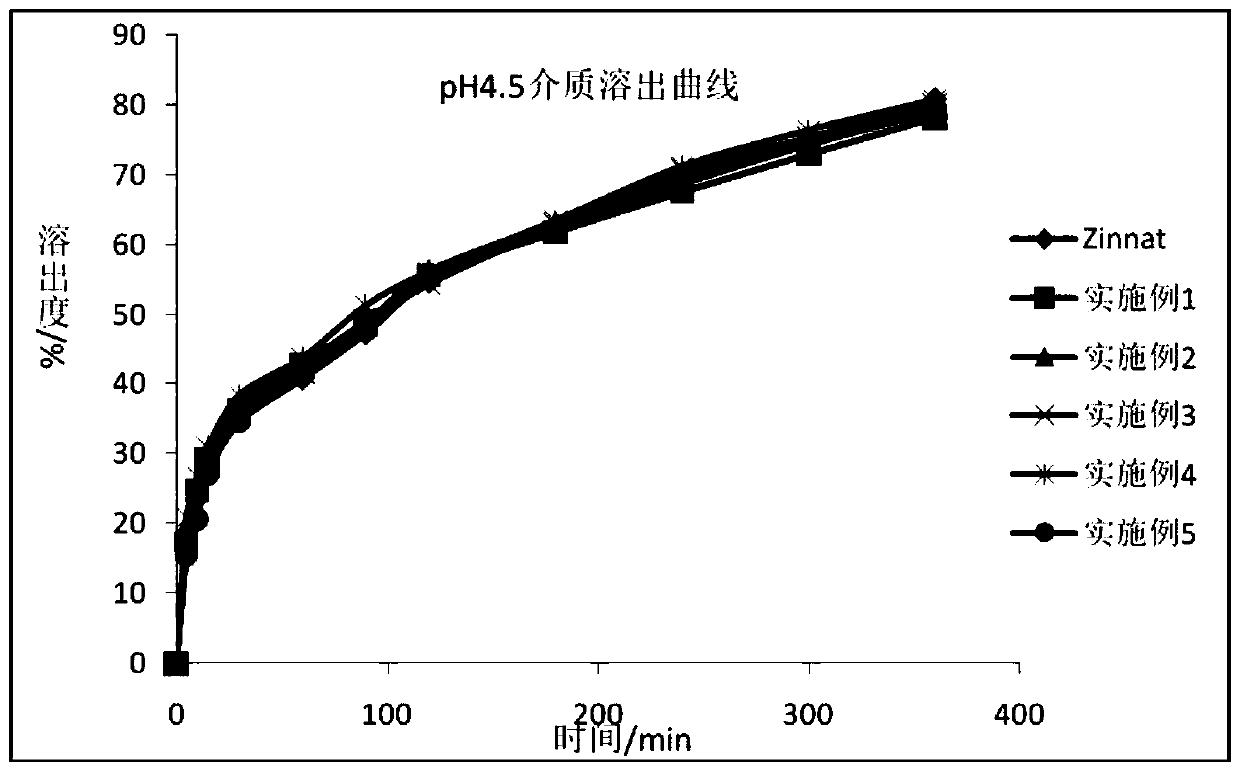
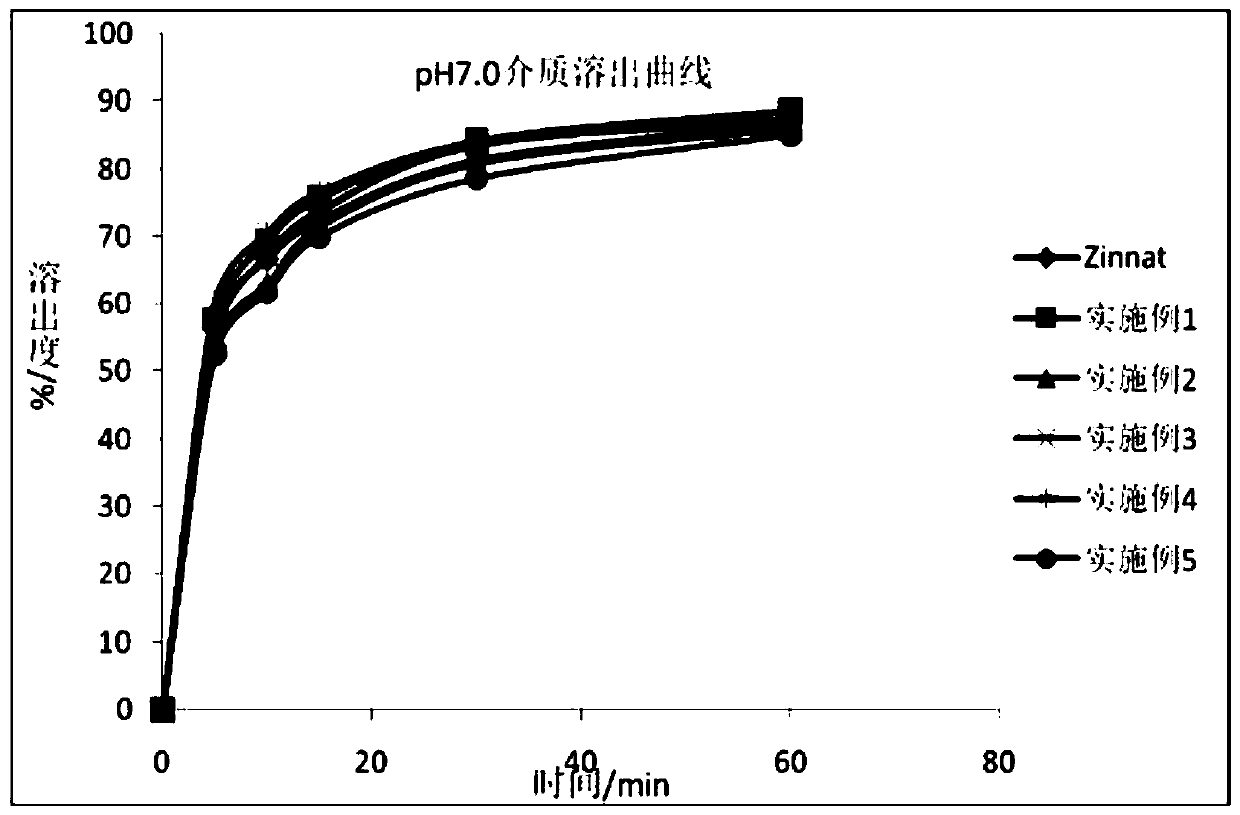
Cefuroxime axetil granule and preparation method thereof
A technology of cefuroxime axetil and granules, which is applied in the direction of pharmaceutical formulations, medical preparations with non-active ingredients, medical preparations containing active ingredients, etc., and can solve problems such as agglomeration, bitter aftertaste of preparations, poor dispersion, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0038] The present invention also provides the preparation method of the cefuroxime axetil granule described in above-mentioned technical scheme, comprises the following steps:
[0039] The fluidized bed granulation process is adopted to obtain drug-containing granules;
[0040] Coating the solution prepared by the taste-masking coating ingredients on the drug-containing particles by using a fluidized bed coating process to form the taste-masking coating ingredients;
[0041] Spray into the essence solution to obtain the cefuroxime axetil granules.
[0042] The invention adopts a fluidized bed granulation process to obtain drug-containing granules. In the present invention, the parameters of the fluidized bed granulation process preferably include: air inlet temperature 65-75°C, atomization pressure 0.1-0.3Mpa, material temperature 35-45°C, drying for 30 minutes after the granulation is completed, and outlet pot, through an 80-mesh sieve for whole grains.
[0043] The inven...
Embodiment 1
[0047] Drug-containing granulation prescription:
[0048]
[0049] Taste-masking coating prescription:
[0050]
[0051] Fragrance water solution prescription:
[0052] Flavor 5g
[0053] water 100g
[0054] Preparation Process:
[0055] ①Drug-containing granules:
[0056] Cefuroxime axetil and sucrose were crushed through a 140-mesh sieve; aspartame was crushed through a 200-mesh sieve;
[0057] Weigh 40g of hypromellose (5cps), add it to about 240g of water, stir to disperse evenly, add the remaining purified water and stir until dissolved, and make a 5% (w / w) hypromellose aqueous solution;
[0058] Weigh cefuroxime axetil, sucrose, and aspartame and mix them evenly, then place them in a fluidized bed granulation coating machine, spray the prepared hypromellose aqueous solution into the top spray for granulation, and the equipment parameters are as follows:
[0059] Air inlet temperature: set to 65°C, atomization pressure: 0.1Mpa, so that the temperature of the m...
Embodiment 2
[0069] prescription:
[0070] Drug-containing granulation prescription:
[0071]
[0072] Taste-masking coating prescription:
[0073]
[0074] Fragrance water solution prescription:
[0075] Flavor 5g
[0076] water 100g
[0077] Preparation Process:
[0078] ①Drug-containing granules:
[0079] Cefuroxime axetil and mannitol were crushed through a 140-mesh sieve; neotame was crushed through a 200-mesh sieve;
[0080] Weigh 40g of hypromellose (5cps), add it into about 240g of hot water, stir and disperse evenly, add the remaining purified water and stir until dissolved, and prepare a 5% (w / w) hypromellose aqueous solution;
[0081] Weigh cefuroxime axetil, mannitol, and neotame and mix them evenly, then place them in a fluidized bed granulation coating machine, spray the prepared hypromellose aqueous solution into the top spray for granulation, and the equipment parameters are as follows:
[0082] Air inlet temperature: set to 65°C, atomization pressure: 0.1Mpa, ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com