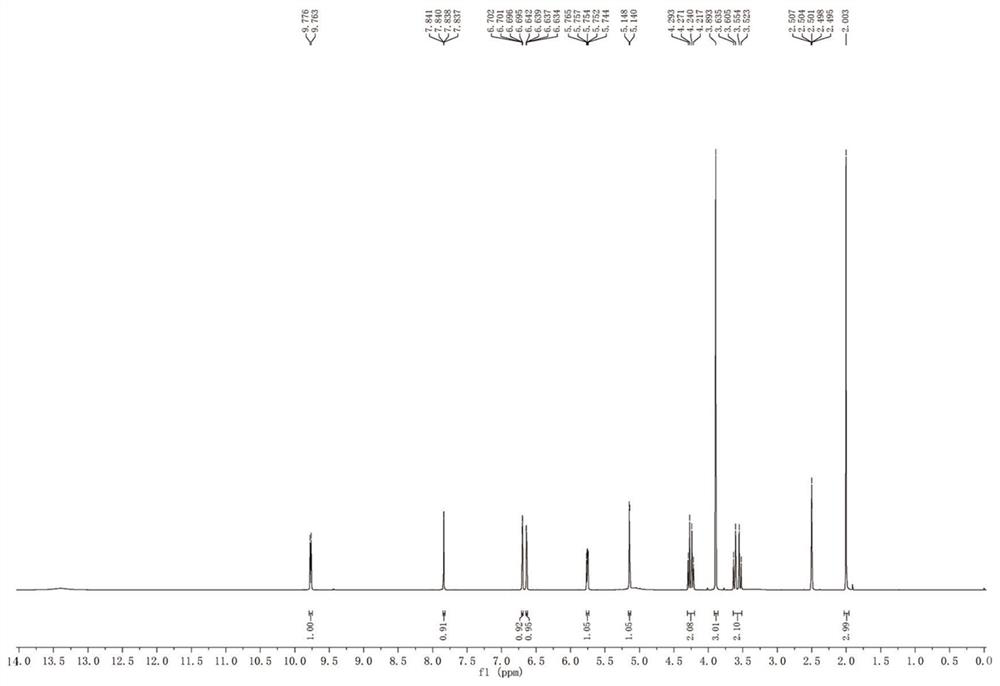
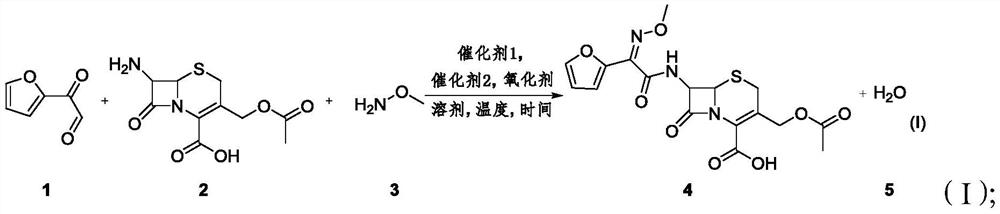
A method for synthesizing 3-decarbamoyl-acetyl-cefuroxin compound
A technology of cefuroxime acid and aminocephalosporanic acid, which is applied in the field of organic compound synthesis, can solve the problems of waste water, waste liquid, many process steps, increased cost and environmental protection pressure, and achieve low cost and environmental protection pressure and simple process steps Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0039] In a 25mL reaction vessel, the weighed PdCl 2 [(tBu) 2 P(OH)] (5.0% mol) was put in, airtight, and replaced with nitrogen three times; then 2-(2-furyl)-2-oxo-acetaldehyde (1.2equiv.) and 7-aminocephalosporin Acid (0.2mmol, 1.0equiv.) was dissolved in 2-methyltetrahydrofuran (0.1M) except the water, and then, the solution was injected into a closed reaction vessel; then the weighed tert-butanol peroxide ( 2.0equiv.) into; fully stirred, and reacted at 45°C for 2.5 hours; then, the weighed pivalic acid (5.0%mol) was put into the reaction vessel, and then the weighed pivalic acid was kept at 45°C. A good 40%wt methoxyamine (2.0euqiv.) aqueous solution was slowly dripped dropwise into a closed reaction vessel for 2.5 hours; the residue of 7-aminosporanic acid was detected by high performance liquid chromatography, and the After the reaction, cool the system to room temperature; wash, extract the organic phase, and use flash column chromatography to remove the target produ...
Embodiment 2
[0042] In a 25mL reaction vessel, the weighed Pd(tBu 3 ) 2 (5.0%mol) was put in, airtight, replaced with nitrogen 3 times; then 2-(2-furyl)-2-oxo-acetaldehyde (1.2equiv.) and 7-aminocephalosporanic acid (0.2mmol, 1.0equiv.) was dissolved in 2-methyltetrahydrofuran (0.1M) except water, and then the solution was poured into a closed reaction vessel; then weighed tert-butanol peroxide (2.0equiv.) into; fully stirred, and reacted for 3 hours at 45°C; then, the weighed pivalic acid (5.0%mol) was put into the reaction vessel, and then the weighed 40%wt Methoxyamine (2.0euqiv.) aqueous solution was slowly dripped into the airtight reaction vessel drop by drop for 3 hours; the residue of 7-aminosporanic acid was detected by high performance liquid chromatography, and after the detection reaction was finished, the Cool the system to room temperature; wash, extract the organic phase, and use flash column chromatography to remove the target product. The stationary phase of the chromato...
Embodiment 3
[0044] In a 25mL reaction vessel, the weighed PdCl 2 (dppf) (10.0% mol) and iodobenzene acetate (2.5equiv.) were put in, airtight, and replaced with nitrogen for 3 times; then 2-(2-furyl)-2-oxo-acetaldehyde (1.2equiv. ) and 7-aminocephalosporanic acid (0.2mmol, 1.0equiv.) were dissolved in tetrahydrofuran (0.1M) except water, and then the solution was poured into a closed reaction vessel; fully stirred and reacted at 50°C for 3 hour; then, the weighed acetic acid (8% mol) is put into the reaction vessel, and at a temperature of 50° C., then the weighed 40% wt methoxyamine (2.5euqiv.) aqueous solution is slowly, Drop by drop into a closed reaction container for 3 hours; detect the residue of 7-aminosporanic acid by high performance liquid chromatography, after the detection reaction is completed, cool the system to room temperature; wash, extract the organic phase, and use a rapid Column chromatography goes out target product, and the stationary phase of chromatographic column...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com