Cefuroxime axetil capsule and preparation method thereof
A technology of cefuroxime axetil and capsules, which is applied in the field of cefuroxime axetil capsules and its preparation, can solve problems affecting product quality and clinical application, unqualified drug content uniformity, excessive content of drug degradation products, etc., and achieve time reduction , low dissolution rate and improved dissolution rate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] Embodiment 1 (1000 grains)
[0035] Prepare according to the formula in Table 1
[0036] Table 1 Embodiment 1 formula
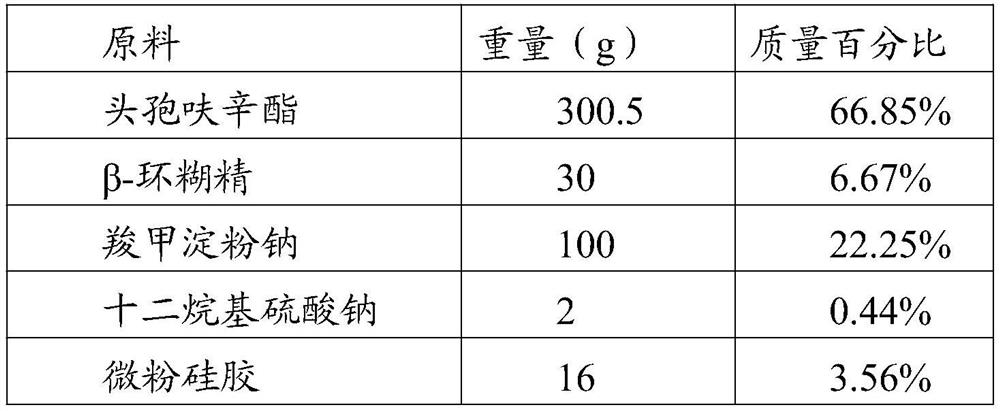
[0037] raw material Weight (g) mass percentage cefuroxime axetil 150.25 66.85% Lactose SuperTab11SD 15 6.67% Carboxymethyl Starch Sodium 50 22.25% Sodium dodecyl sulfate 1 0.44% Micropowder silica gel 8 3.56% Magnesium stearate 0.5 0.22%
[0038] The preparation method is as follows:
[0039] 1. Material pretreatment: pass sodium lauryl sulfate, micropowder silica gel, and magnesium stearate through an 80-mesh sieve; pass cefuroxime axetil and lactose through a 60-mesh sieve, and set aside;
[0040] 2. Pre-mixing: Weigh sodium lauryl sulfate, micropowder silica gel, and magnesium stearate and mix for 5 minutes, pass through a 60-mesh sieve, and sieve three times to obtain mixture A;
[0041] 3. Mixing: Add cefuroxime axetil to mixture A, mix for 5 minutes, add sodium starch glycolate and lac...
Embodiment 2
[0043] Embodiment 2 (1000 grains)
[0044] Prepare according to the formula in Table 2
[0045] Table 2 Example 2 formula
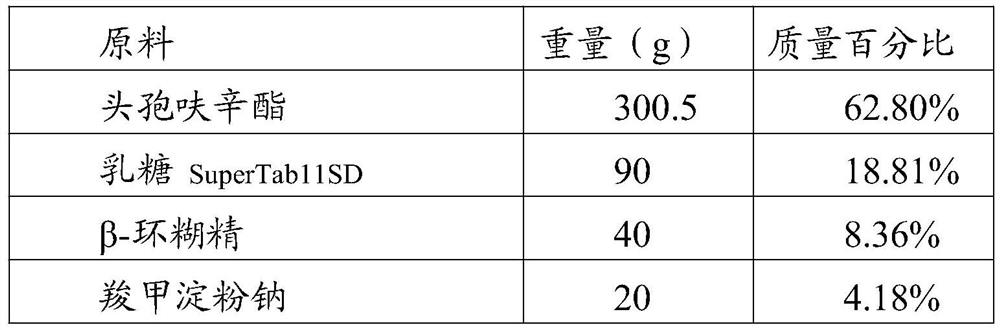
[0046] raw material Weight (g) mass percentage cefuroxime axetil 150.25 66.85% β-cyclodextrin 15 6.67% Carboxymethyl Starch Sodium 50 22.25% Sodium dodecyl sulfate 1 0.44% Micropowder silica gel 8 3.56% Magnesium stearate 0.5 0.22%
[0047] The preparation method is as follows:
[0048] 1. Material pretreatment: pass sodium lauryl sulfate, micropowder silica gel, and magnesium stearate through a 80-mesh sieve; pass cefuroxime axetil and β-cyclodextrin through a 60-mesh sieve; set aside;
[0049] 2. Pre-mixing: Weigh sodium lauryl sulfate, micropowder silica gel, and magnesium stearate and mix for 5 minutes, pass through a 60-mesh sieve, and sieve three times to obtain mixture A;
[0050] 3. Mixing: Add cefuroxime axetil to mixture A, mix for 5 minutes, add sodium starch glycolate and β-cyclodextr...
Embodiment 3
[0052] Embodiment 3 (1000 grains)
[0053] Prepare according to the formula in Table 3
[0054] Table 3 Example 3 formula
[0055] raw material Weight (g) mass percentage cefuroxime axetil 150.25 71.29% Microcrystalline Cellulose PH301 28 13.29% Lactose SuperTab11SD 15 7.12% Croscarmellose Sodium 10 4.74% Sodium dodecyl sulfate 1 0.47% Micropowder silica gel 6 2.85% Magnesium stearate 0.5 0.24%
[0056] The preparation method is as follows:
[0057] 1. Material pretreatment: pass sodium lauryl sulfate, micropowder silica gel, and magnesium stearate through an 80-mesh sieve; pass cefuroxime axetil, lactose, and microcrystalline cellulose through a 60-mesh sieve; set aside;
[0058] 2. Pre-mixing: Weigh sodium lauryl sulfate, micropowder silica gel, and magnesium stearate and mix for 5 minutes, pass through a 60-mesh sieve, and sieve three times to obtain mixture A;
[0059] 3. Mixing: Add cefuroxime axetil to ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com