Method for analyzing content of acetyl bromide in cefuroxime axetil
A technology of cefuroxime axetil and an analysis method, applied in the field of analysis and detection, can solve the problems of inability to directly measure the content of cefuroxime axetil acetyl bromide, the influence of drug safety, etc., and achieves the advantages of short reaction time, reduced pollution and simple operation. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0051] Linear, linear range analysis:
[0052] Proceed as follows:
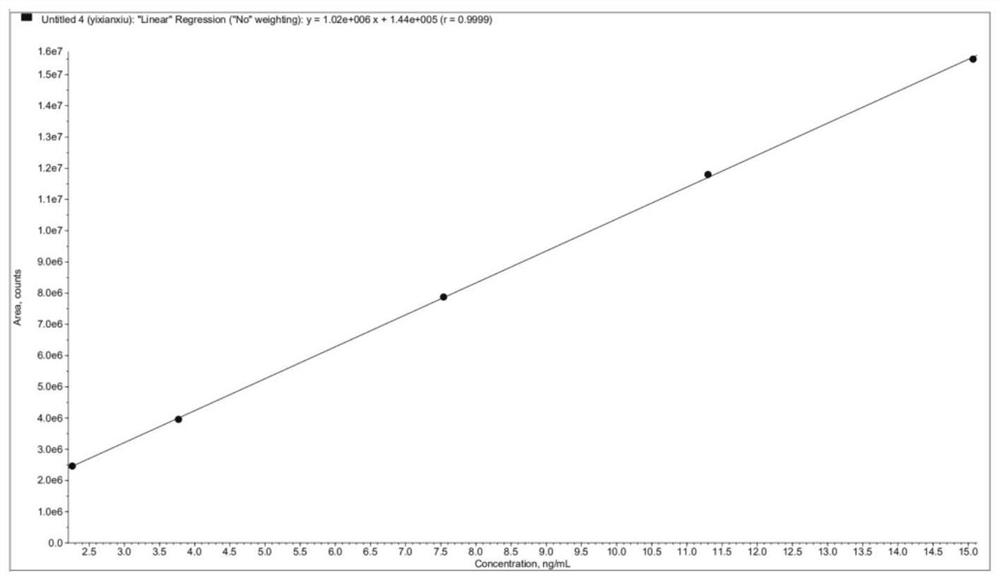
[0053] Accurately measure an appropriate amount of acetyl bromide derivative solution of 75ng / ml, and dilute with acetonitrile to obtain linear solutions of 2.25ng / ml, 3.75g / ml, 7.5ng / ml, 11.25ng / ml, and 15ng / ml; , and then analyze the linear solution in turn, and draw a regression curve with the concentration of each linear solution versus the peak area.
[0054] Results Discussion:
[0055] Acetyl bromide standard curve as figure 1 As shown, Y=1.02e 6 X+1.44e 5 , r=0.9999; there is a linear relationship between the concentration of 2.26ng / ml~15.07ng / ml (corresponding to the limit concentration of 30%~200%), and the linear correlation coefficient r=0.9999.
Embodiment 2
[0057] Precision Analysis:
[0058] Proceed as follows:
[0059] (1) Blank test solution: Take about 10 mg of cefuroxime axetil, weigh it accurately, put it in a 5 ml sample preparation bottle, add 200 μl of 1 μg / ml 2,4-dinitrophenylhydrazine solution, vortex to dissolve, and derivatize After reacting for 1 h, add 1800 μl of acetonitrile, shake well, and obtain. Prepare 3 copies in the same way.
[0060](2) 100% standard-added test solution: Take about 10 mg of cefuroxime axetil, weigh it accurately, put it in a 5ml sample bottle, add 200 μl, 75ng of 1 μg / ml 2,4-dinitrophenylhydrazine solution successively 200 μl of reference substance derivatization solution per ml, vortex to dissolve, after 1 hour of derivatization reaction, add 1600 μl of acetonitrile, shake well, and obtain. Prepare 6 copies in the same way.
[0061] Results Discussion:
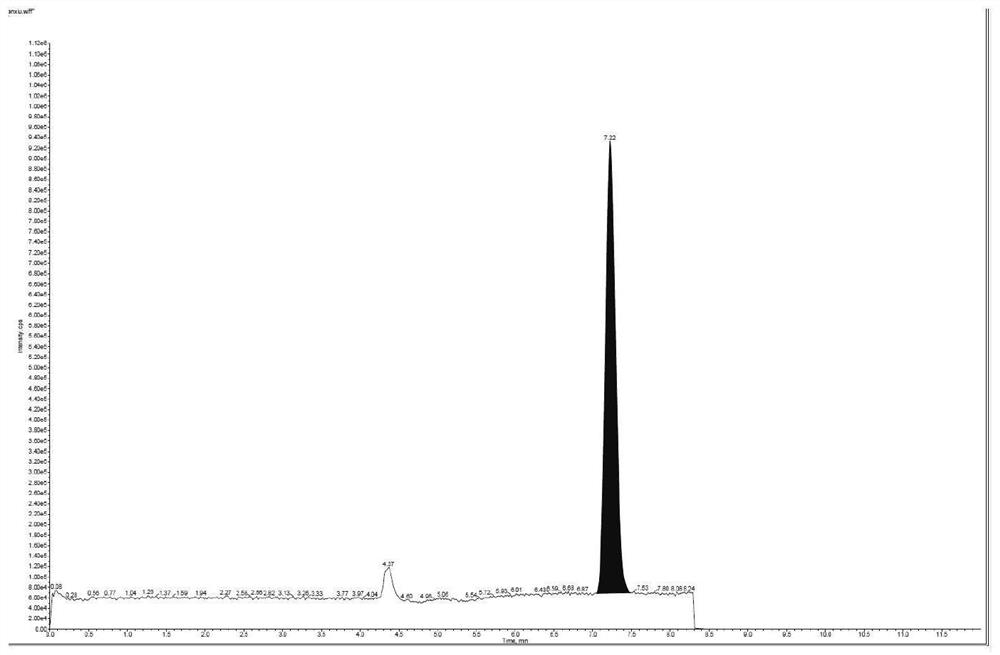
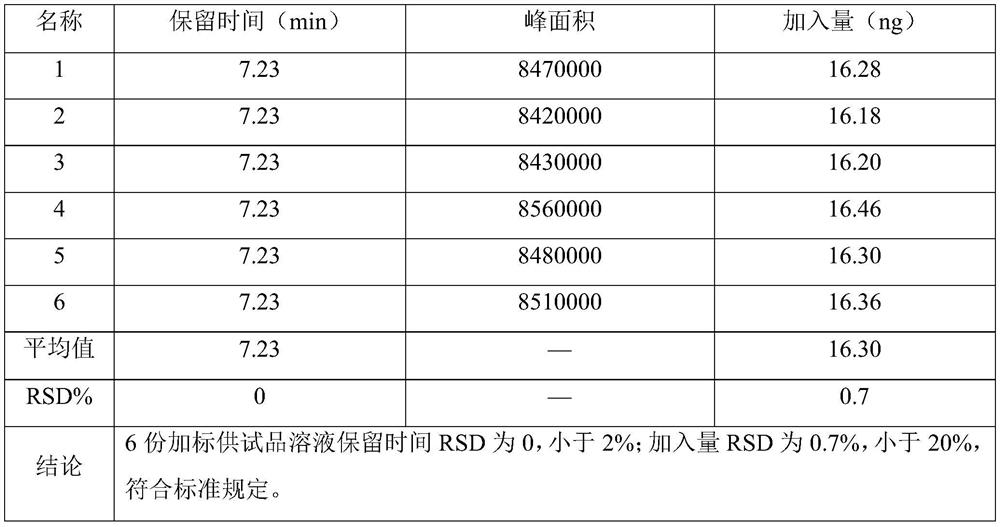
[0062] The precision inspection results of acetyl bromide are shown in Table 1 respectively.
[0063] Table 1 Results of precision...
Embodiment 3
[0066] Accuracy analysis:
[0067] Proceed as follows:
[0068] (1) Blank test solution: Take about 10 mg of cefuroxime axetil, weigh it accurately, put it in a 5 ml sample preparation bottle, add 200 μl of 1 μg / ml 2,4-dinitrophenylhydrazine solution, vortex to dissolve, and derivatize After reacting for 1 h, add 1800 μl of acetonitrile, shake well, and obtain. Prepare 3 copies in the same way.
[0069] (2) 50% standard-added test solution: Take about 10 mg of cefuroxime axetil, weigh it accurately, put it in a 5 ml sample preparation bottle, add 200 μl of 1 μg / ml 2,4-dinitrophenylhydrazine solution, 75ng / ml reference substance derivatization solution 100 μl, vortex to dissolve, after 1 hour of derivatization reaction, add 1700 μl of acetonitrile, shake well, and obtain. Prepare 3 copies in the same way.
[0070] (3) 100% standard-added test solution: Take about 10 mg of cefuroxime axetil, weigh it accurately, put it in a 5 ml sample preparation bottle, add 200 μ l of 1 μ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com