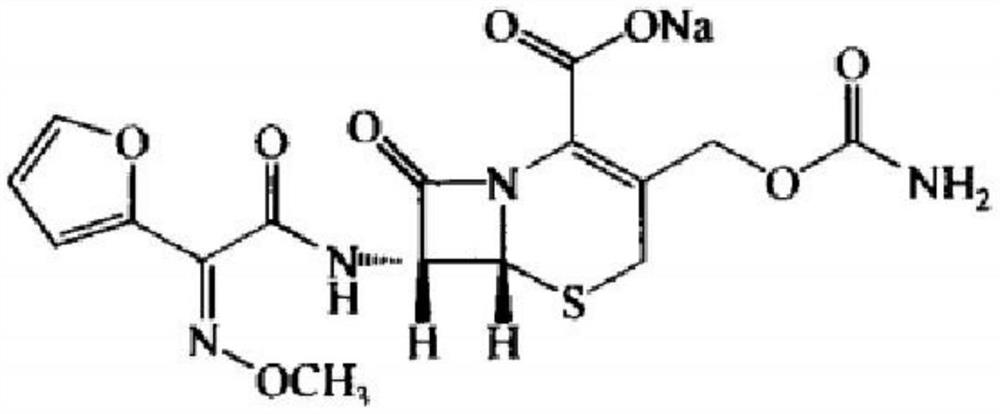
Preparation method of cefuroxime acid
A technology of cefuroxime acid and cefuroxime sodium, which is applied in the field of medicine, can solve the problems of high production cost, and achieve the effects of less impurities, easy operation and good stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0035] A preparation method of cefuroxime acid, characterized in that: using unqualified cefuroxime sodium as a raw material, put it into a dissolving agent and stir to dissolve it, then add an adsorbent to stir, after filtering, use an acidic reagent to crystallize to obtain cefuroxime bitter.
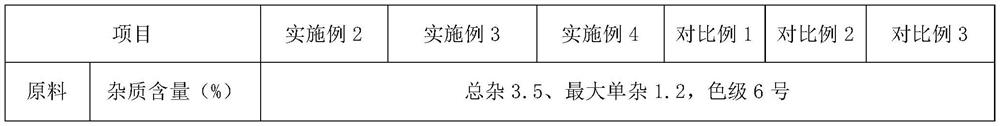
[0036] The quality index of cefuroxime sodium satisfies any one or a combination of total impurity ≤ 5.0%, maximum single impurity ≤ 2.0%, or solution color ≤ No. 7.
[0037] Including the following steps:
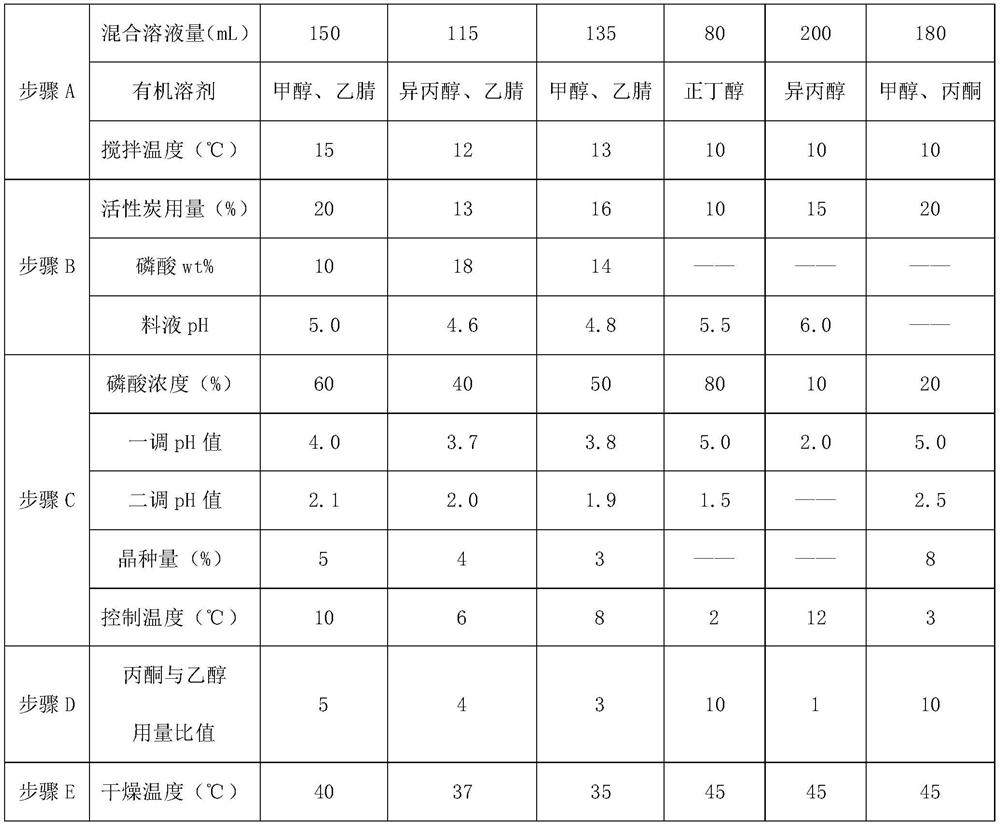
[0038] A. prepare mixed solution, control temperature, drop into unqualified cefuroxime sodium product, stir and dissolve to dissolve clear, obtain cefuroxime sodium solution liquid;
[0039] The mixed solution is selected from the combination of methanol, Virahol, acetonitrile and water; the volume and water ratio of each organic solvent in the mixed solution is 1:1:2; The gram-to-weight ratio is 20-30:1, and the temperature is controlled at 10-15°C during stirring.
[0040]B....
Embodiment 1
[0047] Add 100mL of methanol-isopropanol-water mixed solution into the dissolution tank, start stirring, control the temperature at 10°C, add 5 g of crude cefuroxime sodium, stir to dissolve, add 1 g of activated carbon loaded with 10% phosphoric acid, and check the pH value in the tank. When the pH drops to 4.5, filter, add 30% phosphoric acid to the filtrate dropwise to pH 3.6, add 0.15g cefuroxime acid, grow the crystal for 1 hour, continue to add 30% phosphoric acid dropwise until the pH is 1.9, stop, cool down to 5°C, filter and crystallize liquid, washed the wet powder with 15mL of acetone ethanol (3:1), placed in a decompression drying oven at 30°C and dried until the water content was qualified, and 4.61g of cefuroxime acid product was obtained.
Embodiment 2~4
[0049] Embodiments 2-4 have the same production process steps as in Embodiment 1, the difference is the selection of process parameters, as shown in Table 1 below.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com