A kind of environment-friendly preparation method of the intermediate of cefuroxime acid
A technology for cefuroxime acid and intermediates, which is applied in the field of environmental protection preparation of intermediates, can solve problems such as large environmental impact, unsatisfactory yield and quality, and achieves reduction of solvent residues, reduction of the amount of chloride salts and phosphates, Responsive effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0024] The present invention relates to a kind of environment-friendly preparation method of the intermediate of cefuroxime acid, comprising:
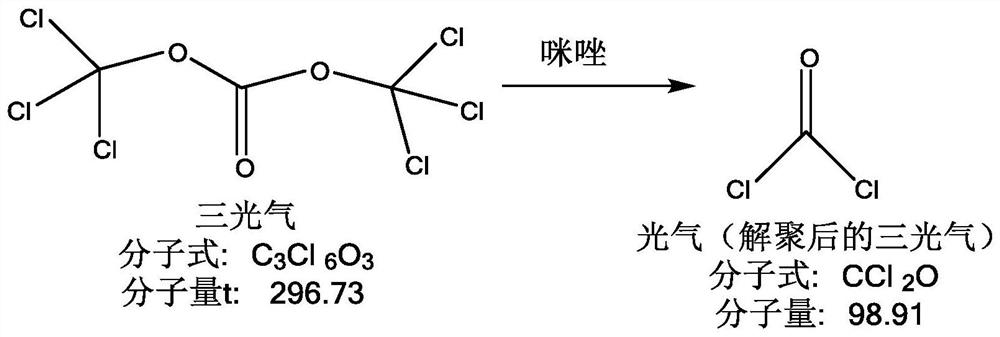
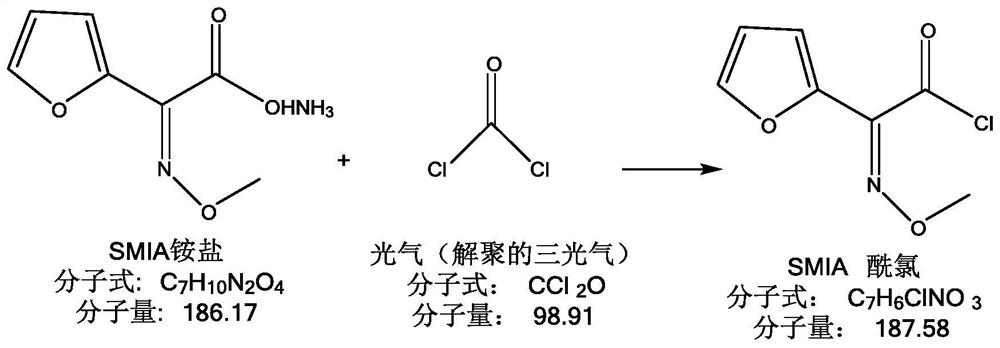
[0025] A, prepare SMIA acid chloride solution: as figure 1 As shown, dissolve triphosgene in an organic solvent, stir at low temperature, add imidazole at low temperature after fully dissolving, stir at low temperature, and depolymerize triphosgene; figure 2 As shown, add methoxyimidofuran ammonium acetate (SMIA ammonium salt) and continue the low-temperature reaction. After the reaction is completed, add purified water to wash, separate liquid, discard the water phase, and keep the organic phase to obtain methoxyimidefuran B Acid chloride solution (SMIA acid chloride solution); organic solvents include but not limited to esters, alcohols, hydrocarbons, ketones, aldehydes, amines, nitriles, ethers, etc., such as dichloromethane, acetone, ether, methanol, etc. Wait;
[0026] Among them, the CAS registration number of triphosgene: 323...
Embodiment 1
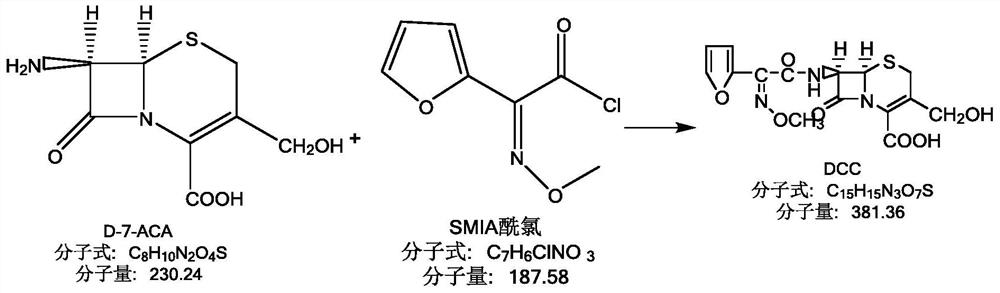
[0045] Dissolve 8g of triphosgene in 70ml of dichloromethane, and control the temperature at -20-0°C under stirring. After fully dissolving, add 0.3g of imidazole, control the temperature at -5°C and add in batches, and the addition will be completed within about 5 minutes. The reaction was stirred for 90-120 minutes. Then add 13 g of SMIA ammonium salt, control the temperature at -20-0° C. and react for about 1-2 hours. HPLC monitoring shows that SMIA is less than 0.5%, and the reaction is basically completed. Add 40ml of cold water to wash twice, separate the liquid, discard the water phase, and keep the organic phase, which is the SMIA acid chloride solution with a purity of 99.2%. In another reaction bottle, add 100ml of pure water, lower the temperature to below 5°C, add 13.5g of D-7-ACA, add dropwise 10% sodium hydroxide solution to dissolve, pay attention to control pH = 7-10, after dissolution, control below Store at 5°C for later use. Add the SMIA acid chloride solu...
Embodiment 2
[0047] Dissolve 2.8g of triphosgene in 70ml of dichloromethane solution, and control the temperature at 0-20°C while stirring. After fully dissolving, add 0.014g of imidazole, control the temperature at -5°C and add in batches, and complete the addition within about 5 minutes. The reaction was stirred for 30-90 minutes. Then add 2.8 g of SMIA ammonium salt, control the temperature at about -20-0° C. and react for about 30-60 minutes. HPLC monitoring shows that SMIA is less than 0.5%, and the reaction is basically completed. Add 40ml of cold water to wash twice, separate the liquid, discard the water phase, and keep the organic phase, which is the SMIA acid chloride solution with a purity of 98.9%. In another reaction bottle, add 1000ml of pure water, lower the temperature to below 5°C, add 1g of D-7-ACA, add 10% sodium hydroxide solution dropwise to dissolve, pay attention to control pH = 7-10, control it below 5 after dissolution ℃ for later use. Add the SMIA acid chloride ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com