Preparation method and application of cefuroxime sodium
A technology of cefuroxime sodium and cefuroxime acid, which is applied in the field of medicine, can solve the problems of unsatisfactory stability and color of cefuroxime sodium, unsatisfactory dissolving effect of cefuroxime, large amount of water and acetone, and achieve residual solvent less, lower production costs, and reduced dosage
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
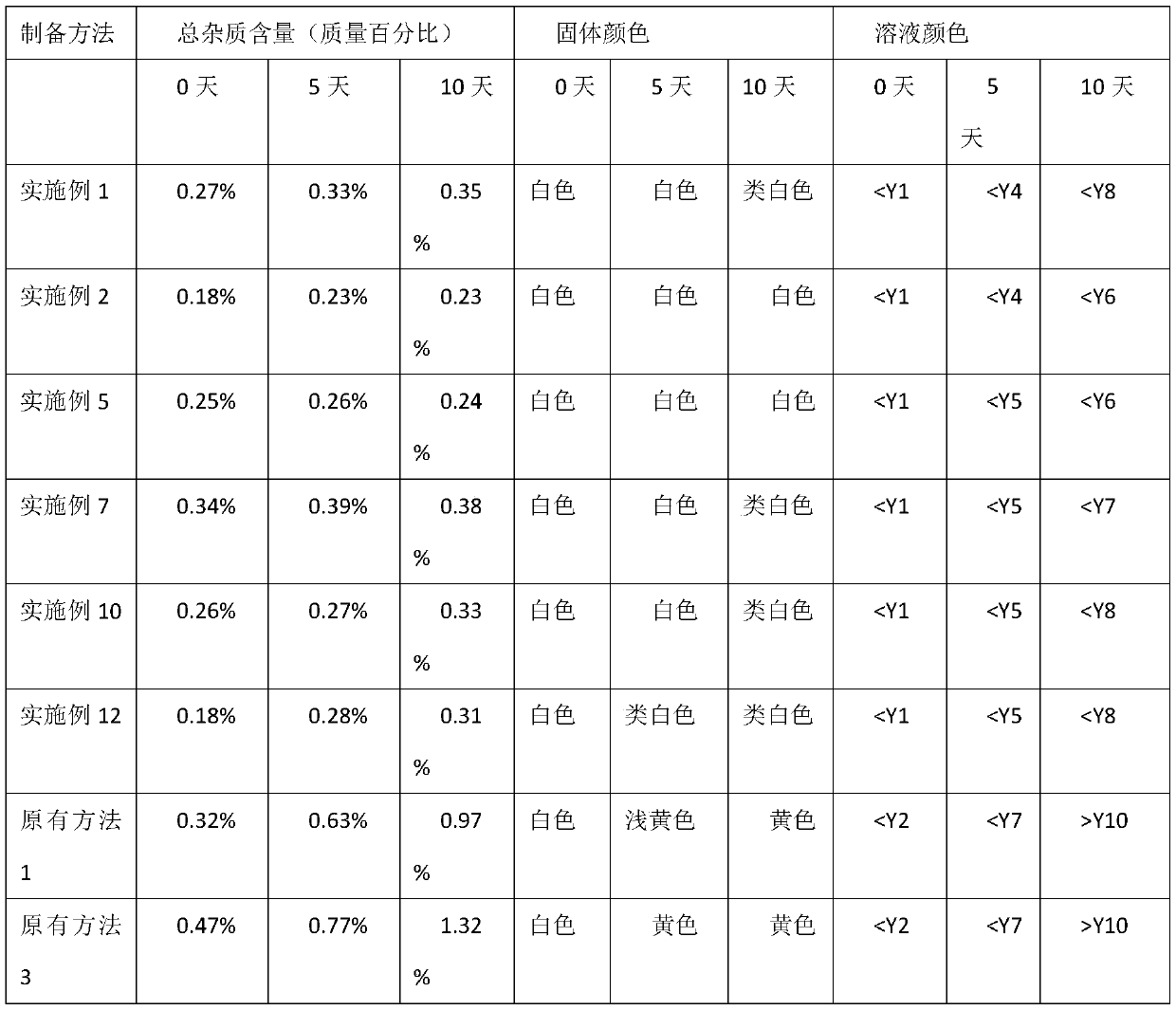
Image
Examples
Embodiment 1
[0035] Dissolve 10.0g of cefuroxime acid in a mixed solution of 7ml of water and 70ml of acetone, stir until dissolved, add 1.0ml of 10.0% sodium sulfite aqueous solution, then add 0.5g of activated carbon and stir for 30 minutes, filter to remove carbon, and mix with appropriate amount of acetone and water Wash the charcoal with the solution, combine the filtrate and the charcoal washing solution, control the temperature at 30°C, stir the solution of 5.0g 60% sodium lactate and 50ml ethanol into the above combined solution, stir for 30 minutes after the addition, then add 100ml of acetone dropwise, continue stirring 60 minutes; filter, collect, wash the crystals, and vacuum-dry at 35° C. to obtain 10.10 g of stable cefuroxime sodium, with a yield of 96.0%.
[0036] Wherein the calculation method of yield is: yield (%)=mass of cefuroxime sodium obtained in experiment / theoretical quality of cefuroxime sodium. Specifically, the molecular weight of cefuroxime acid is 424.39, the ...
Embodiment 2
[0038] Dissolve 10.0g of cefuroxime acid in a mixed solution of 7ml of water and 70ml of acetone, stir until dissolved, add 1.0ml of 10.0% sodium metabisulfite aqueous solution, then add 0.5g of activated carbon and stir for 30 minutes, filter to remove charcoal, and use appropriate amount of acetone and water Wash the charcoal with the mixed solution, combine the filtrate and the charcoal washing solution, control the temperature at 30°C, stir the solution of 5.0g 60% sodium lactate and 30ml methanol into the above combined solution, stir for 30 minutes after the addition, then add 100ml of acetone dropwise, continue Stir for 60 minutes; filter, collect, wash the crystals, and vacuum-dry at 35° C. to obtain 9.8 g of stable cefuroxime sodium, with a yield of 93.2%.
Embodiment 3
[0040] Dissolve 10.0g of cefuroxime acid in a mixed solution of 7ml of water and 70ml of acetone, stir until dissolved, add 0.20ml of 10.0% sodium metabisulfite aqueous solution, then add 0.5g of activated carbon and stir for 30 minutes, filter to remove charcoal, and add appropriate amount of acetone and water Wash the charcoal with the mixed solution, combine the filtrate and the charcoal washing solution, control the temperature at 30°C, stir and add 12.0g of 20.0% sodium acetate aqueous solution into the above combined solution, stir for 30 minutes after the addition, then add 50ml of acetone dropwise, and continue to stir for 60 minutes; filter, collect, wash the crystals, and vacuum-dry at 35°C to obtain 10.45 g of stable cefuroxime sodium, with a yield of 99.3%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com