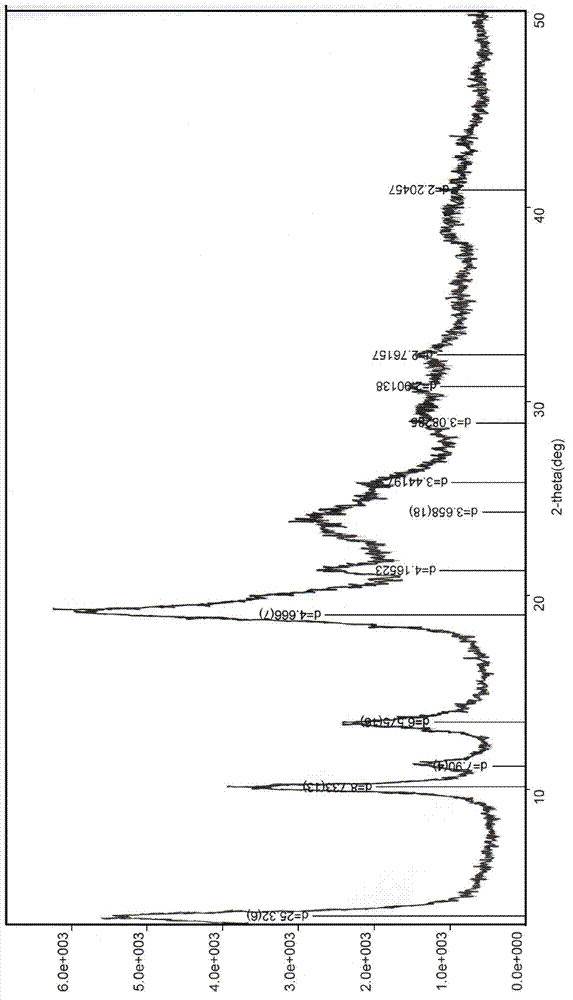
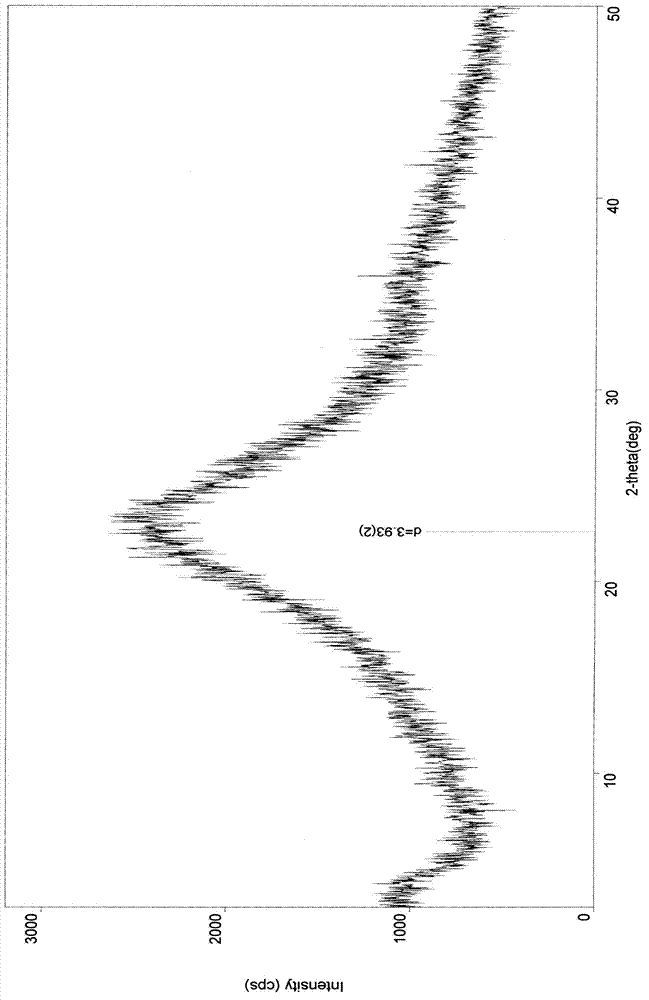
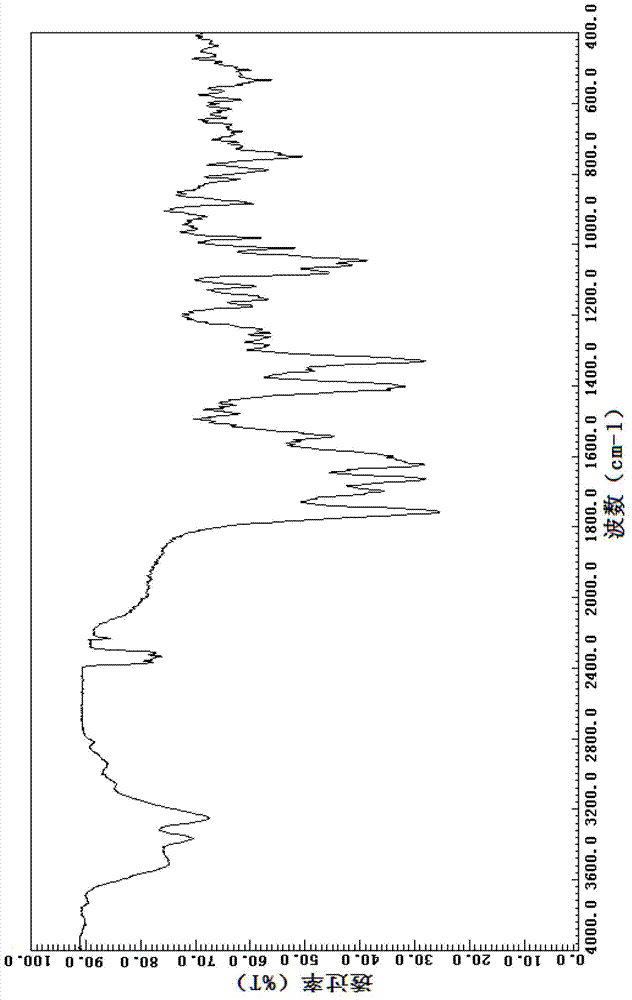
Cefuroxime sodium crystal compound and composition powder injection thereof
A technology of cefuroxime sodium and cefuroxime acid, which is applied in the field of cefuroxime sodium new crystal compound and its composition powder injection, can solve the problem of complex production process of cefuroxime sodium, poor water solubility of cefuroxime sodium, and clinical application Inconvenient and other problems, to achieve the effect of fast dissolution, high solubility, and convenient clinical use
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0039] 1. Prescription:
[0040] Cefuroxime Acid (95%) 1052g
[0041] Sodium bicarbonate for injection (98%) 202g
[0042] Water for injection 1578ml
[0043] 2. Production process:
[0044] According to aseptic operation requirements, 202g sodium bicarbonate was dissolved in 1000ml water, 1052g cefuroxime acid was dissolved in 578ml water to form sodium bicarbonate solution and cefuroxime acid solution, and sodium bicarbonate solution was slowly added in the cefuroxime acid solution (above Control the water temperature of the two solutions at 5-8°C), and keep stirring for 30 minutes. After the reaction is complete, add 0.1% activated carbon and stir to adjust the pH to 6.5-7.5. After filtering through 0.45 μm and 0.22 μm filter membranes, put them into a large freeze-drying plate (Liquid level height 3-4cm), immediately sent to a vacuum freeze-drying box to freeze-dry, first lower the temperature of the plate layer to below -40°C, and then send it into the product, within ...
Embodiment 2
[0051] 1. Prescription
[0052] Cefuroxime Acid (95%) 1052g
[0053] Sodium bicarbonate for injection (98%) 202g
[0054] Water for injection 1578ml
[0055] 2. Production process:
[0056] According to aseptic operation requirements, 202g sodium bicarbonate was dissolved in 1000ml water, 1052g cefuroxime acid was dissolved in 578ml water to form sodium bicarbonate solution and cefuroxime acid solution, and sodium bicarbonate solution was slowly added in the cefuroxime acid solution (above Control the water temperature of the two solutions at 5-8°C), and keep stirring for 30 minutes. After the reaction is complete, add 0.1% activated carbon and stir to adjust the pH to 6.5-7.5. After filtering through 0.45 μm and 0.22 μm filter membranes, put them into a large freeze-drying plate (Liquid level height 3-4cm), immediately sent to a vacuum freeze-drying box for freeze-drying, first lowered the temperature of the plate layer to below -45°C, and then sent it to the product, with...
Embodiment 3
[0059] Preparation of cefuroxime sodium composition powder injection: get 750g of cefuroxime sodium amorphous compound sterile powder prepared in Example 1, mannitol sterile powder 6g, mix well, according to specifications 2.5g / bottle (according to cefuroxime sodium Sodium meter), aseptically divided into antibiotic glass bottles, stoppered and capped, and the finished product is packaged and put into storage and sent for inspection.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com