A kind of cefuroxime sodium raw material and injection and preparation method thereof
A technology of cefuroxime sodium and cefuroxime acid, which is applied in the field of medicine and can solve problems such as rapid growth and poor control of related substances 2
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0039] Embodiment 1 prepares cefuroxime sodium raw material of the present invention
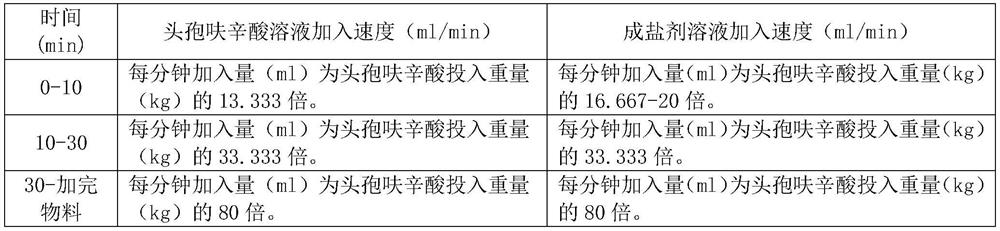
[0040] (1) Preparation of cefuroxime acid solution: add methanol (50L), dehydrated ethanol (98L) and water (2L) respectively in a clean stainless steel batching tank A, stir and control the liquid temperature in the stainless steel tank A to 10 -15°C, then add cefuroxime acid (15kg) weighed in advance, control the temperature at 10-15°C and completely dissolve to obtain a cefuroxime acid solution, and set aside;
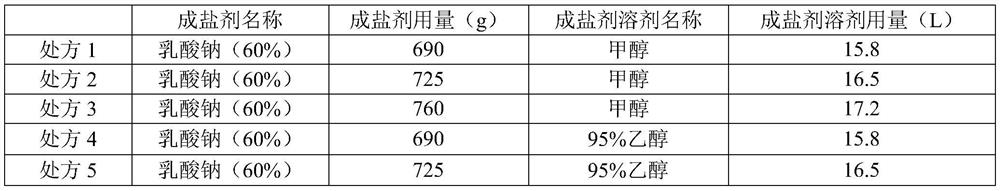
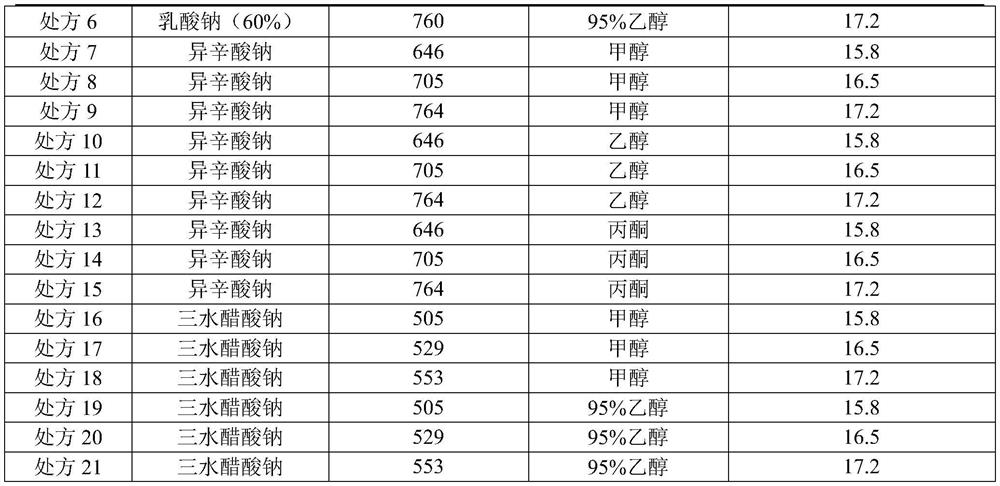
[0041] (2) Preparation of salt-forming agent solution: Measure and add 95% ethanol (150L) into the clean stainless steel batching tank B, stir and control the liquid temperature in the stainless steel tank B to 10-15°C, and then add the mixture that has been weighed in advance Sodium acetate trihydrate (5.29kg), temperature control 10-15 ℃ and after dissolving completely, obtain salt-forming agent solution, standby;
[0042] (3) Salt-forming reaction: Methanol (10L), absolute etha...
Embodiment 2
[0044] Embodiment 2 prepares cefuroxime sodium raw material of the present invention
[0045] (1) Preparation of cefuroxime acid solution: add methanol (55L), absolute ethanol (108L) and water (2L) into a clean stainless steel batching tank respectively, stir and control the liquid temperature in the stainless steel tank to 10-15 ℃, then add cefuroxime acid (15kg) weighed in advance, after temperature control 10-15 ℃ and completely dissolved, cefuroxime acid solution is obtained for subsequent use;
[0046] (2) Preparation of salt-forming agent solution: Measure and add 95% ethanol (165L) respectively in a clean stainless steel batching tank, stir and control the liquid temperature in the stainless steel tank to 10-15°C, then add the pre-weighed three Water sodium acetate (5.29kg), temperature control 10-15 ℃ and after completely dissolving, obtain salt-forming agent solution, standby;
[0047] (3) Salt-forming reaction: Methanol (11L), absolute ethanol (21.6L), water (0.4L),...
Embodiment 3
[0049] Embodiment 3 prepares cefuroxime sodium raw material of the present invention
[0050] (1) Preparation of cefuroxime acid solution: Methanol (60L), dehydrated ethanol (118L) and water (2L) were metered into clean stainless steel batching tank A respectively, stirred and the liquid temperature in stainless steel tank A was controlled to 10 -15°C, then add cefuroxime acid (15kg) weighed in advance, control the temperature at 10-15°C and completely dissolve to obtain a cefuroxime acid solution, and set aside;
[0051] (2) Preparation of salt-forming agent solution: Measure and add 95% ethanol (180L) into the clean stainless steel batching tank B respectively, stir and control the liquid temperature in the stainless steel tank B to 10-15°C, then add the Sodium acetate trihydrate (5.29kg), temperature control 10-15 ℃ and after dissolving completely, obtain salt-forming agent solution, standby;
[0052] (3), salt-forming reaction: Methanol (12L), absolute ethanol (23.6L), wa...
PUM
| Property | Measurement | Unit |
|---|---|---|
| specific rotation | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com