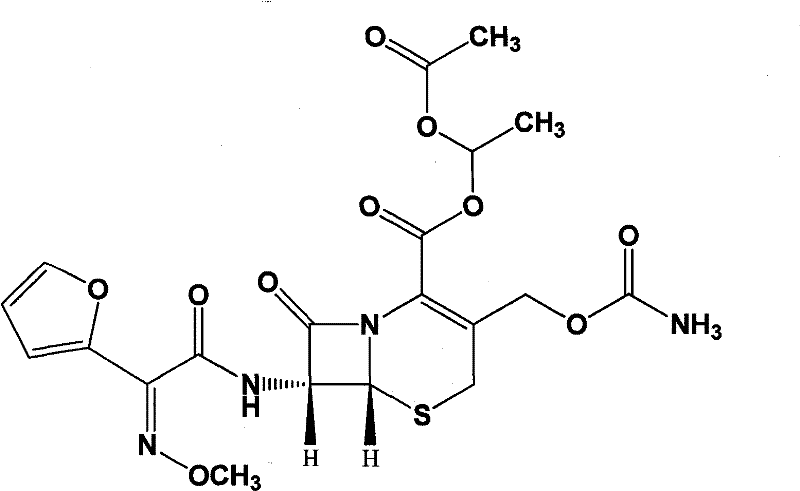
Pharmaceutical composition of cefuroxime axetil for suspension and preparation method thereof
A technology of cefuroxime axetil and dry suspension, which is applied in the direction of pharmaceutical formulations, antibacterial drugs, and medical preparations containing active ingredients, etc. It can solve the problem of inappropriateness, reduce the compliance of patients with clinical medication, and cannot effectively cover up the bitter taste, etc. problem, achieve the effect of improving bitterness and improving compliance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0045] Preparation of cefuroxime axetil dry suspension:
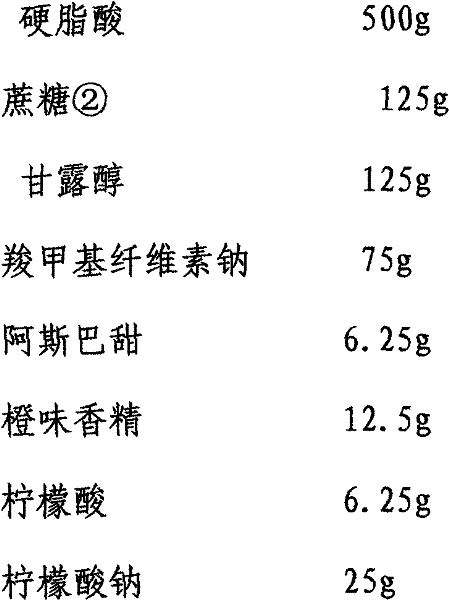
[0046] Quantity per 1000 bags
[0047]
[0048]
[0049] Preparation:
[0050] (1) Pass the raw and auxiliary materials through a sieve of 80 mesh (except stearic acid).
[0051] (2) Mix the prescribed amount of sucrose ① and cefuroxime axetil through an 80-mesh sieve, and mix evenly. Melt the prescribed amount of stearic acid at 55-80°C, add a uniform mixture of cefuroxime axetil and sucrose, stir evenly, cool at room temperature, and pulverize until the particle size is between 30-80 mesh.
[0052] (3) Add the remaining prescription amount of auxiliary materials and mix evenly.
[0053] (4) Divide into dry suspension.
Embodiment 2
[0055] Preparation of cefuroxime axetil dry suspension:
[0056] Quantity per 1000 bags
[0057]
[0058]
[0059] Preparation:
[0060] (1) Pass the raw and auxiliary materials through a sieve of 80 mesh (except stearic acid).
[0061] (2) Mix the prescribed amount of sucrose ① and cefuroxime axetil through an 80-mesh sieve, and mix evenly. Melt the prescribed amount of stearic acid at 55-80°C, add a uniform mixture of cefuroxime axetil and sucrose, stir evenly, cool at room temperature, and pulverize until the particle size is between 30-80 mesh.
[0062] (3) Add the remaining prescription amount of auxiliary materials and mix evenly.
[0063] (4) Divide into dry suspension.
Embodiment 3
[0065] Preparation of cefuroxime axetil dry suspension:
[0066] Quantity per 1000 bags
[0067]
[0068]
[0069] Preparation:
[0070] (1) Pass the raw and auxiliary materials through a sieve of 80 mesh (except stearic acid).
[0071] (2) Mix the prescribed amount of sucrose ① and cefuroxime axetil through an 80-mesh sieve, and mix evenly. Melt the prescribed amount of stearic acid at 55-80°C, add the homogeneous mixture of cefuroxime axetil and sucrose, stir evenly, cool at room temperature, and pulverize until the particle size is between 30-80 mesh.
[0072] (3) Add the remaining prescription amount of auxiliary materials and mix evenly.
[0073] (4) Divide into dry suspension.
[0074] The invention adopts the melting and cooling method to prepare the cefuroxime axetil dry suspension medicinal composition, which effectively covers the bitter taste of the cefuroxime axetil and improves the clinical medication compliance of patients.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com