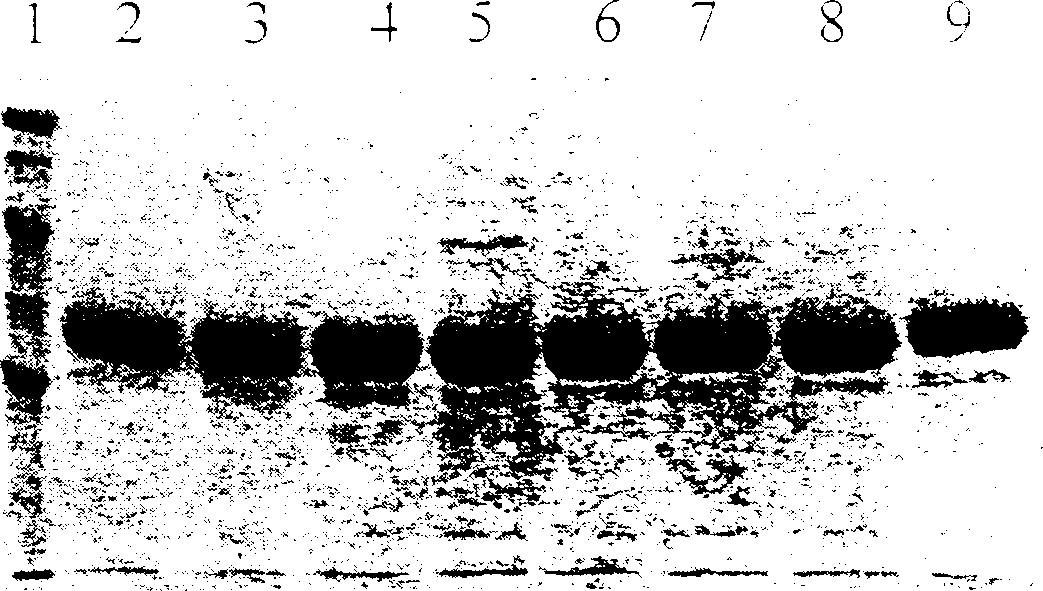
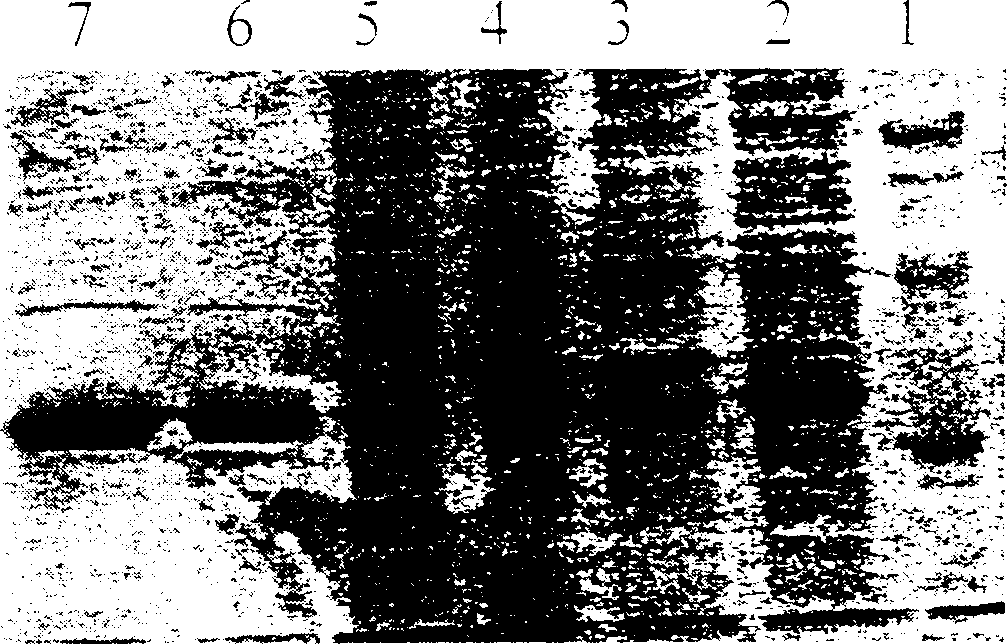Mutation penicillin ring enlargement enzyme and process preparing 7-ADCA using same
An expandase, penicillin technology, applied in the field of penicillin expandase, can solve problems such as low individual activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] Embodiment 1 random mutagenesis
[0033] The cefE gene fragment was excised from pYB4, pYB4 was cloned with the Zero Blunt TOPOPCR Cloning kit from the main chain of pET24a with BamHI-Hind IIIcefE insertion from Streptomyces clavulus, and the cefE gene fragment was treated with 0.8M hydroxylamine at 65°C After 2 hours, it was purified with PCR Clean up-M kit, and then ligated back to the main chain vector. This mutated cefE pool was transformed into BL21(DE3) by electroporation and transformants were selected on plates containing 50 μl / mL kanamycin. Mutated transformants were subjected to activity improvement selection. Example 2 Activity improvement screening
Embodiment 2
[0033] The cefE gene fragment was excised from pYB4, pYB4 was cloned with the Zero Blunt TOPOPCR Cloning kit from the main chain of pET24a with BamHI-Hind IIIcefE insertion from Streptomyces clavulus, and the cefE gene fragment was treated with 0.8M hydroxylamine at 65°C After 2 hours, it was purified with PCR Clean up-M kit, and then ligated back to the main chain vector. This mutated cefE pool was transformed into BL21(DE3) by electroporation and transformants were selected on plates containing 50 μl / mL kanamycin. Mutated transformants were subjected to activity improvement selection. Example 2 Activity improvement screening
[0034] Mutant transformants were grown in 96-well plates with 56.7 μl of LB medium containing kanamycin in each well. 0.1 mM IPTG was then added to each well to indirectly induce DAOCA with shaking for 2 hours at 30°C, followed by an additional 1 hour of shaking after addition of 7 μl of 100 mg / ml lysozyme. The activity of DAOCA was determined by ad...
Embodiment 3
[0036] The wild-type expandase gene (ie, cefE) was cloned by PCR from Streptomyces clavulatus and inserted into the NdeI-HindIII site of pET30a, and the resulting plasmid was named pYS16. The Quick Change Mutagenesis Kit was used to generate site-directed mutagenesis of pYS16. Mutation sites were selected based on the crystal structure of DAOCS (Valegard K. et al., Nature, 394:805-809 (1998)) by selecting residues within 10 Å around the active center using the Swiss-Pdb Viewer (V3.7b2) program. The sites were converted first to Ala residues, then to positively charged residues, hydrophobic residues and sulfur-containing residues. Primers were designed according to the manufacturer's instructions. The resulting mutant was confirmed by DNA sequencing of the plasmid containing the gene, and crude cell extracts were prepared for DAOCS activity assay. Example 4 DAOCS Activity Determination
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com