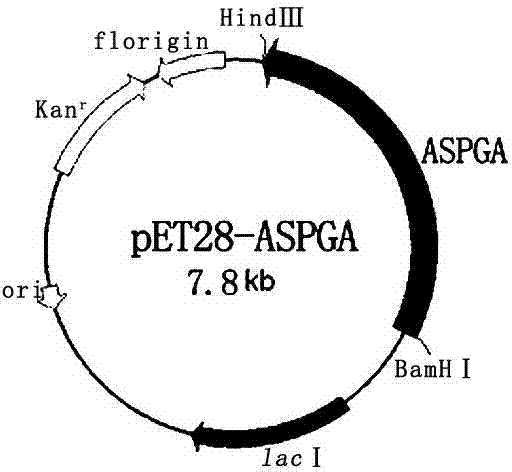
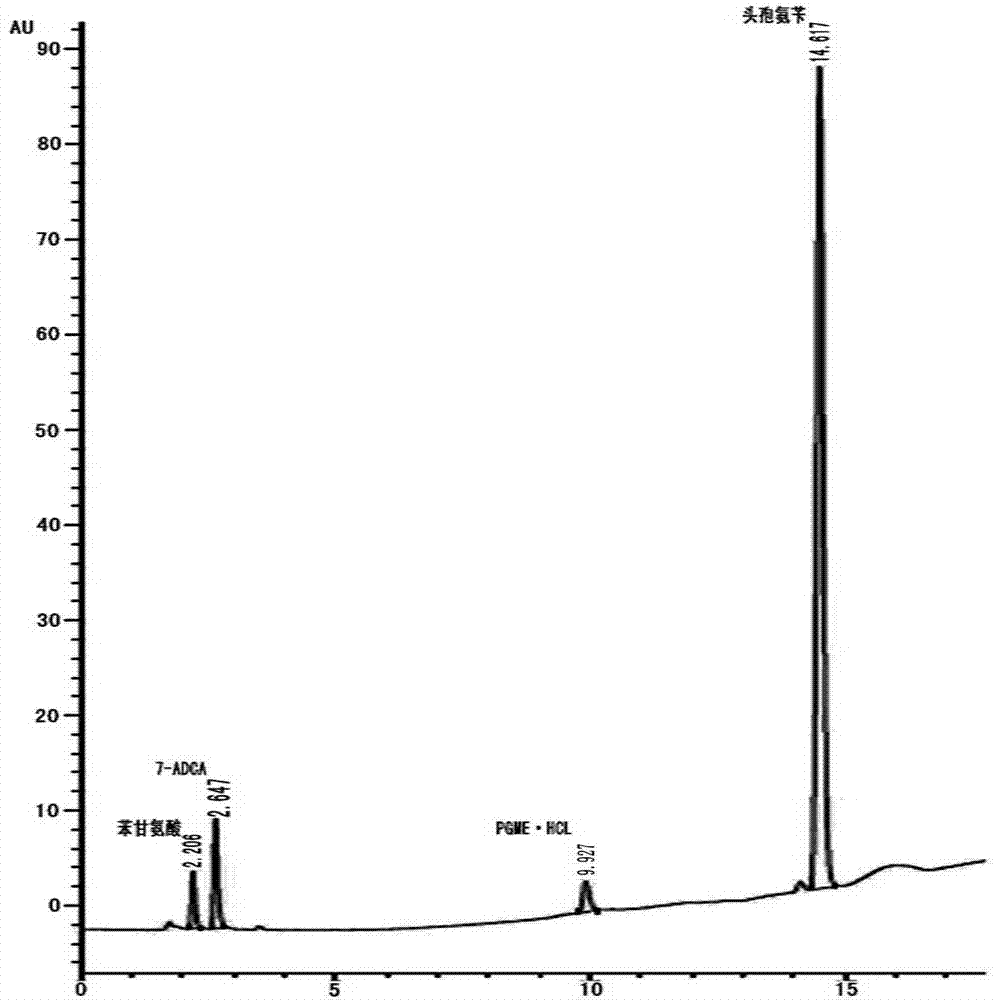
A kind of method for preparing cephalexin
A technology of cephalexin and L-phenylglycine, which is applied in the field of preparation of cephalexin, can solve the problems of serious enzymatic hydrolysis reaction, large amount of side chain feeding, low product purity, etc., and achieve the effect of overcoming reverse reaction, improving product quality and improving conversion rate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0057] 1) Take D-phenylglycine methyl ester sulfate solid (13.0g based on L-phenylglycine methyl ester), slowly add it to the 7-ADCA aqueous solution (100ml, mass concentration 15%), and take 30 to 120 minutes to ensure the system pH The value drops naturally, and ammonia is used to maintain the pH at 6.0-7.2; then 15g of specific penicillin acylase is added, and the reaction is started at 15-25°C.
[0058] 2) The time from the beginning of the reaction to the beginning of turbidity is recorded as A. When the reaction time reaches 2~3×A, start to slowly add 25ml of purified water, which takes 20 to 60 minutes. At the same time, use HPLC during the process of adding purified water. Method to judge the end of the reaction.
[0059] During the above 1)~2) operation, always use ammonia water to keep the pH of the system at 6.0~7.2;
[0060] So far, calculated based on the concentration of 7-ADCA detected by HPLC, the conversion rate of this reaction is 98%.
[0061] 3) After the reaction...
Embodiment 2
[0067] 1) Take D-phenylglycine methyl ester sulfate solid (17.5g based on L-phenylglycine methyl ester) and dissolve it in 40-60ml water to make D-phenylglycine methyl ester sulfate solution, and slowly add it to the 7-ADCA aqueous solution ( 120ml, mass concentration 12.5%), it takes 30-120min to ensure that the PH value of the system naturally drops, and ammonia water is used to maintain the pH at 6.0-7.2; then 20g of specific penicillin acylase is added, and the reaction is started at 15-25°C.
[0068] 2) The time from the beginning of the reaction to the beginning of turbidity is recorded as A. When the reaction time reaches 2~3×A, start to slowly add 40ml of purified water, which takes 20 to 60 minutes. At the same time, use HPLC during the process of adding purified water. Method to judge the end of the reaction.
[0069] During the above 1)~2) operation, always use ammonia water to keep the pH of the system at 6.0~7.2;
[0070] So far, calculated based on the 7-ADCA concentra...
Embodiment 3
[0077] 1) Take D-phenylglycine methyl ester sulfate solid (19.0g based on L-phenylglycine methyl ester) and dissolve it in 10-20ml water to make D-phenylglycine methyl ester sulfate suspension, slowly add it to the 7-ADCA aqueous solution Medium (150ml, mass concentration 10%), it takes 30 to 120 minutes to ensure that the pH of the system naturally drops, and ammonia water is used to maintain the pH at 6.0 to 7.2; then add 30 g of specific penicillin acylase, and start the reaction at 15 to 25°C , And use HPLC method to determine the end of the reaction.
[0078] During the above 1)~2) operation, always use ammonia water to keep the pH of the system at 6.0~7.2;
[0079] So far, calculated based on the 7-ADCA concentration detected by HPLC, the conversion rate of this reaction is 99%.
[0080] 3) After the reaction is terminated, the reaction solution is passed through an 80-mesh screen to obtain penicillin acylase and cephalexin suspensions, and the cephalexin suspension is filtere...
PUM
| Property | Measurement | Unit |
|---|---|---|
| mobile phase | aaaaa | aaaaa |
| mass | aaaaa | aaaaa |
| mass | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com