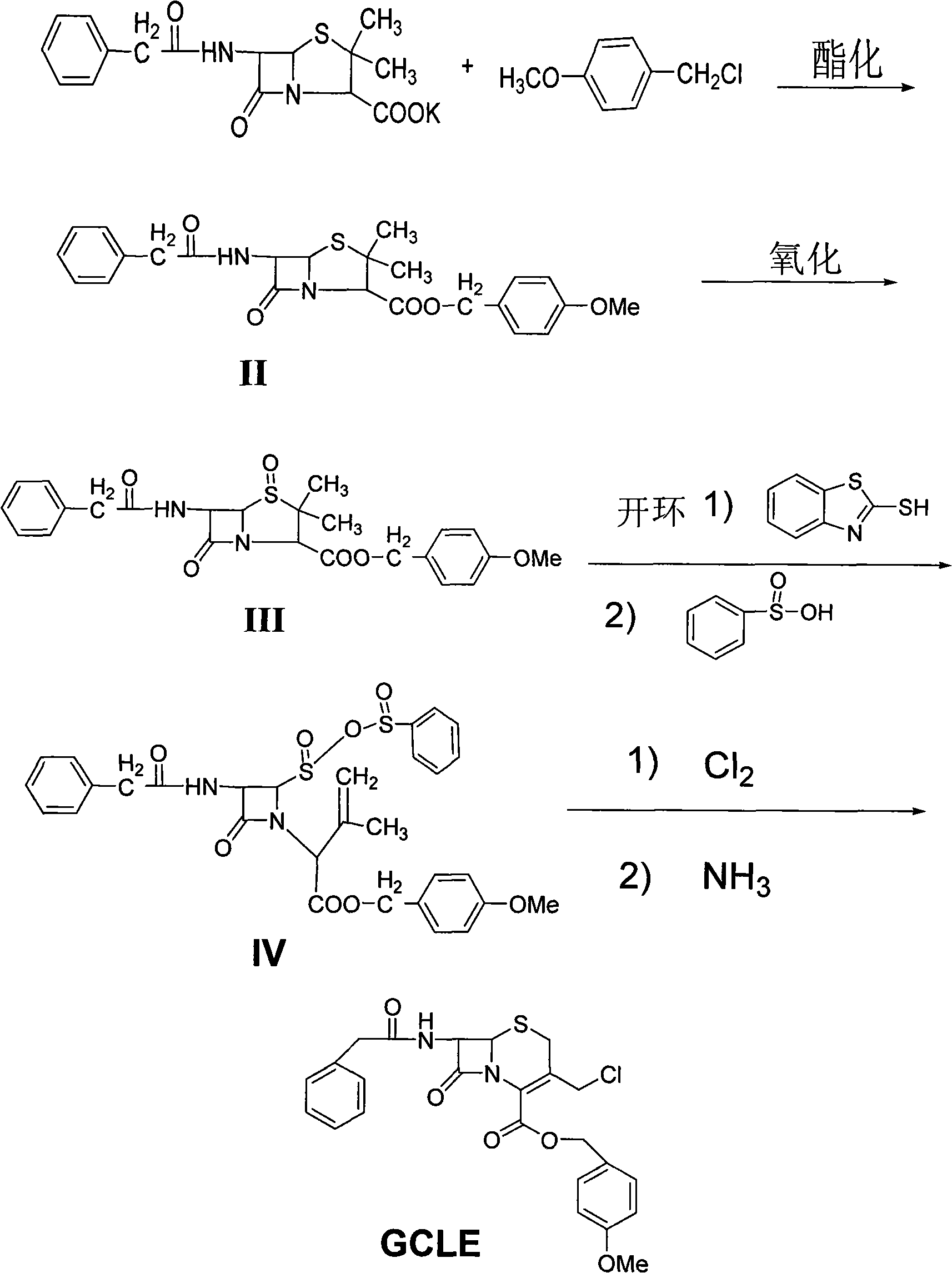
Method for synthesizing 7-phenylacetamide-3-chloromethyl-4-cephalosporanic acid p-methoxybenzyl ester
A technology of cephalosporanic acid and phenylacetamide, which is applied in the field of drug production, can solve problems such as poor quality, high cost, and difficult synthesis technology, and achieve the effects of convenient operation, short synthetic route, and convenient industrial production
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024] (1) Synthesis of penicillin p-methoxybenzyl ester (II)
[0025] Weigh 100g of penicillin industrial salt, add 200mL of DMF and 200mL of purified water to dissolve, add 60g of p-methoxybenzyl chloride and 4g of tetrabutyl bromide, heat to reflux, then react at this temperature for 4 hours, cool to room temperature, and use Extract with 3×150 mL of toluene, combine the extracts, add and dry for 2 hours, the obtained can be directly used in the oxidation reaction without separation, and the yield is 97%.
[0026] (2) Synthesis of penicillin G sulfoxide p-methoxybenzyl ester (III)
[0027] Pour the obtained esterification solution into a three-necked flask, add 100mL of anhydrous methanol, control the temperature at 10-15°C, add 150mL of 15% peracetic acid dropwise, and keep it warm for 20 hours. Wash twice with 200 mL of purified water, then wash twice with 200 mL of 10% sodium bisulfite solution, then dry with anhydrous magnesium sulfate for 2 hours, evaporate the solven...
Embodiment 2
[0033] (1) Synthesis of penicillin p-methoxybenzyl ester (II)
[0034] Weigh 150g of penicillin industrial salt, add 300mL of DMF and 300mL of purified water to dissolve, add 90g of p-methoxybenzyl chloride and 6g of tetrabutyl bromide, heat to reflux, then react at this temperature for 4 hours, cool to room temperature, and use Extract with 3×230 mL of toluene, combine the extracts, add and dry for 2 hours, and the yield is 96.5%.
[0035] (2) Synthesis of penicillin G sulfoxide p-methoxybenzyl ester (III)
[0036] Pour the obtained esterification solution into a three-necked flask, add 150mL of anhydrous methanol, control the temperature at 10-15°C, add 230mL of 15% peracetic acid dropwise, and keep it warm for 20 hours. Wash twice with 300 mL of purified water, then wash twice with 300 mL of 10% sodium bisulfite solution, then dry with anhydrous magnesium sulfate for 2 hours, evaporate the solvent under reduced pressure, add 150 mL of anhydrous methanol to the residue, and...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com