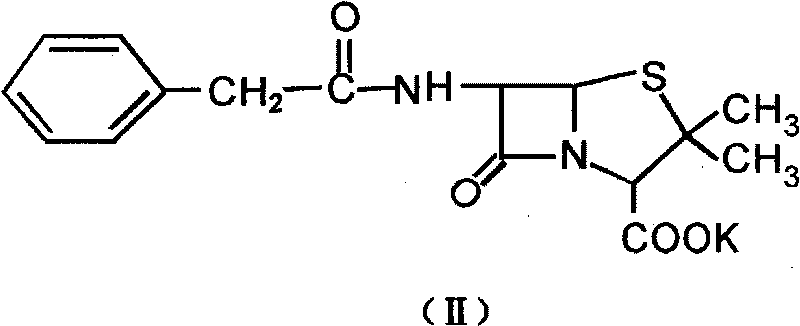
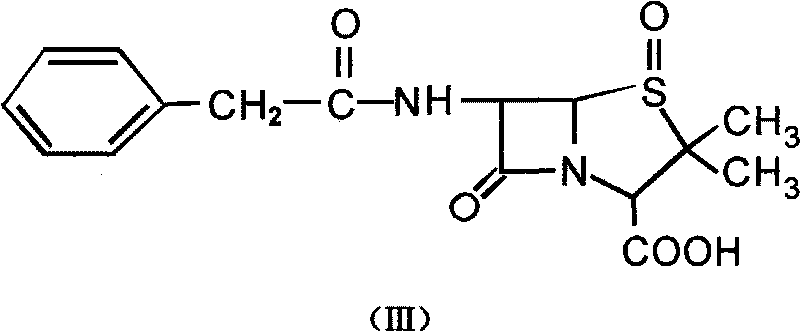
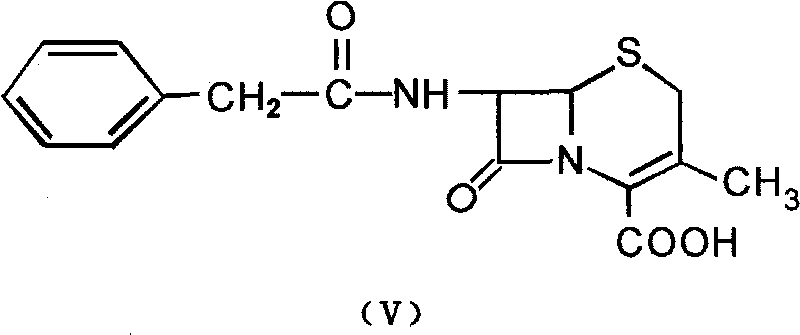
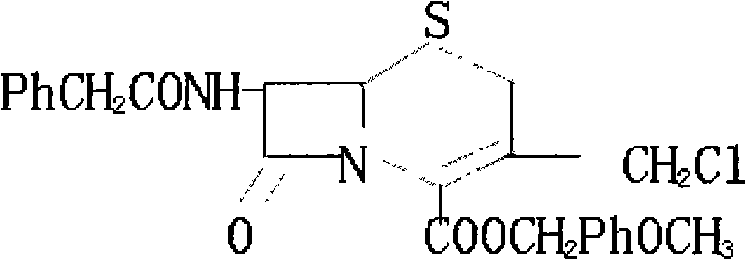
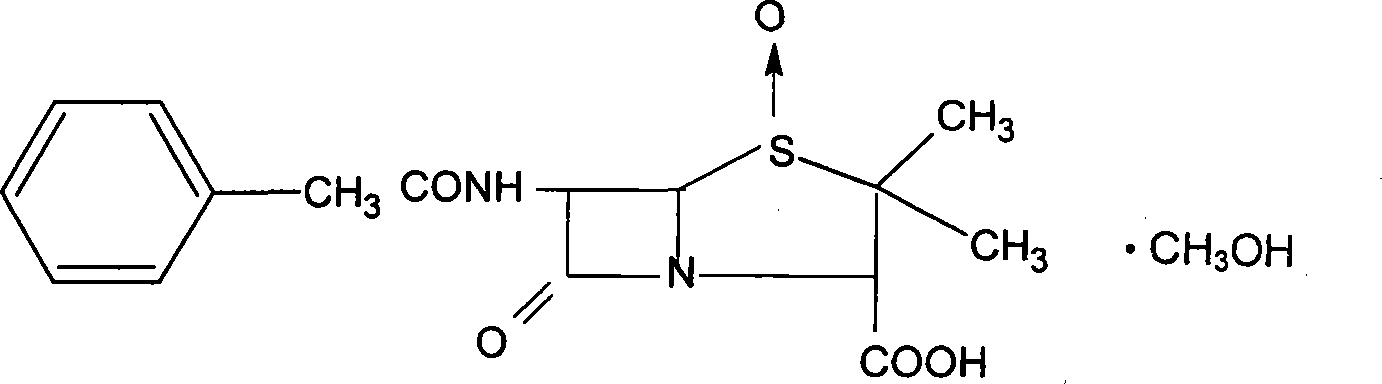
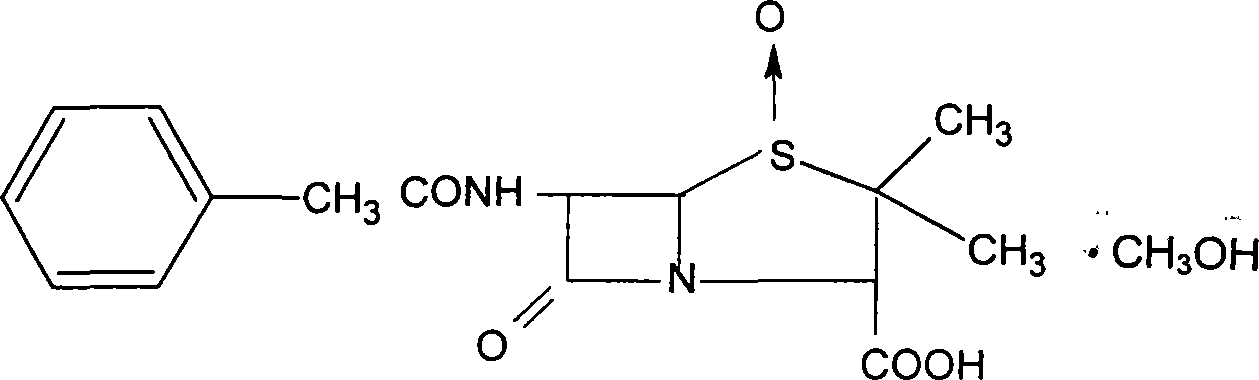
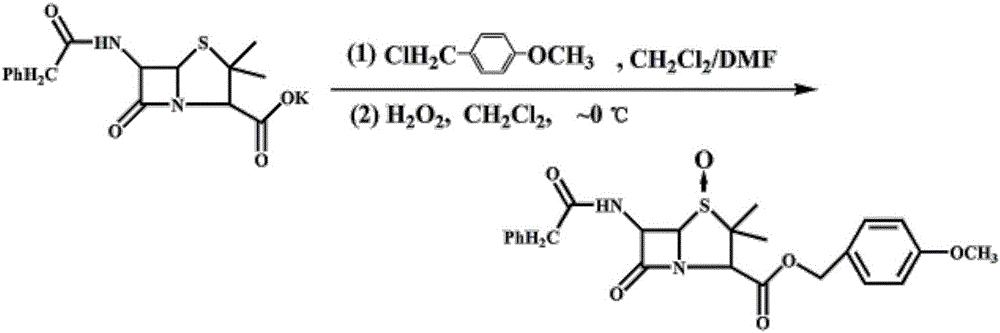
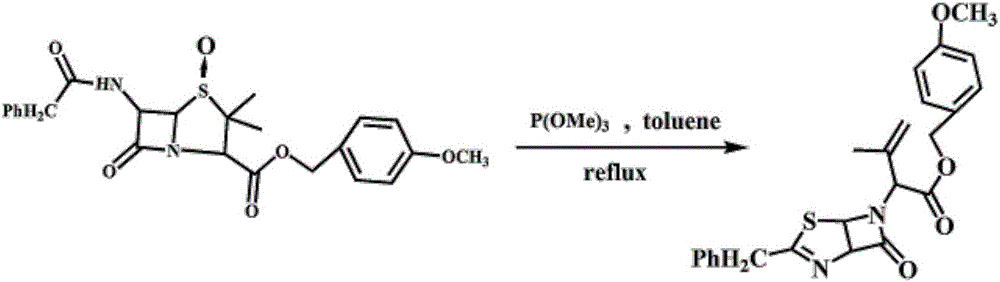
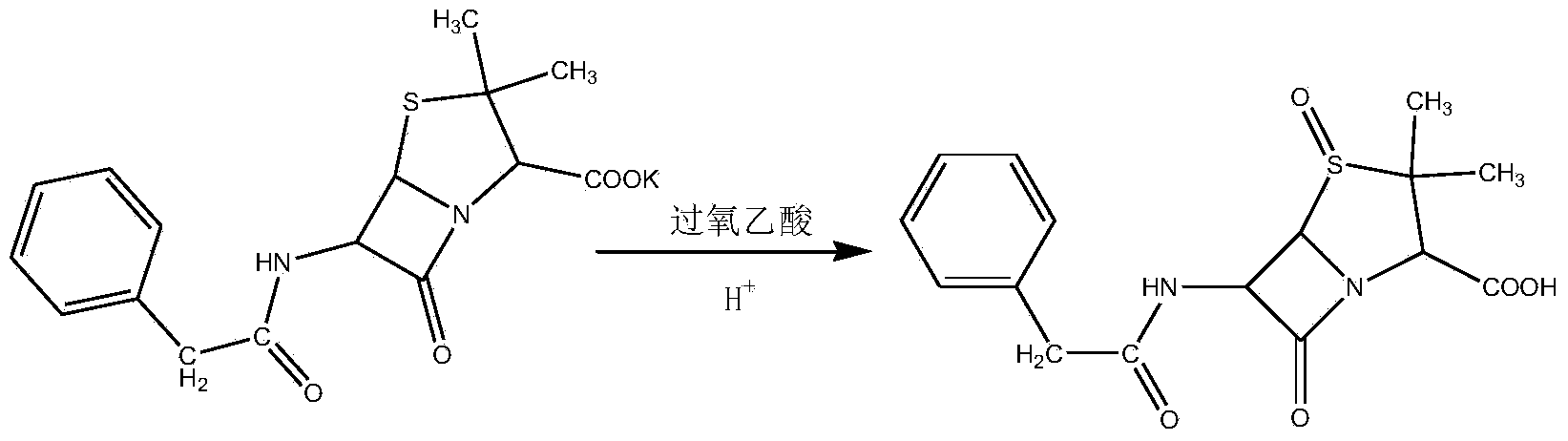
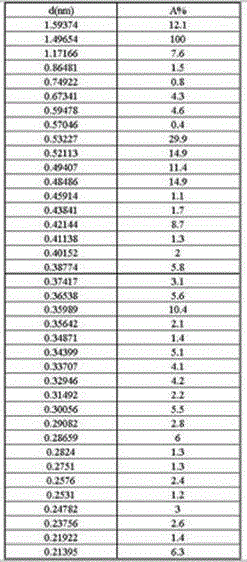
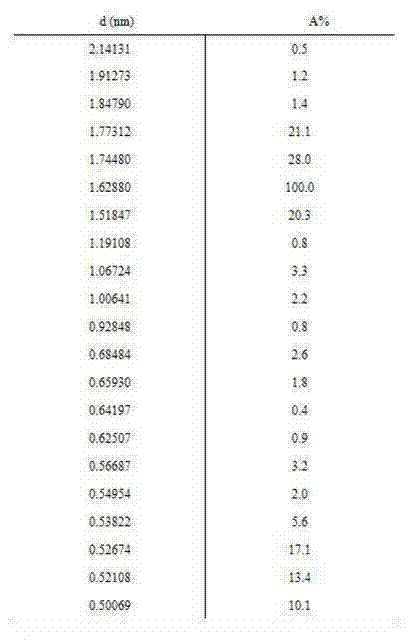
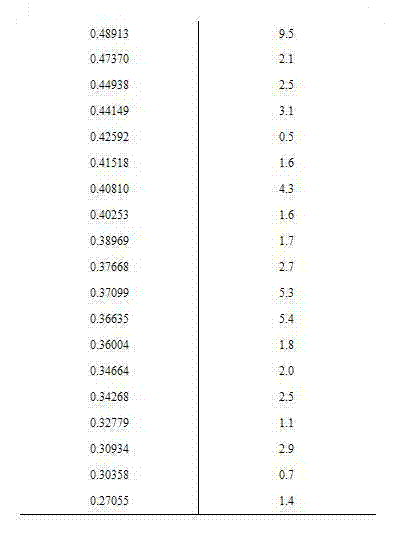
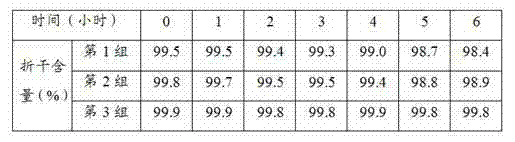
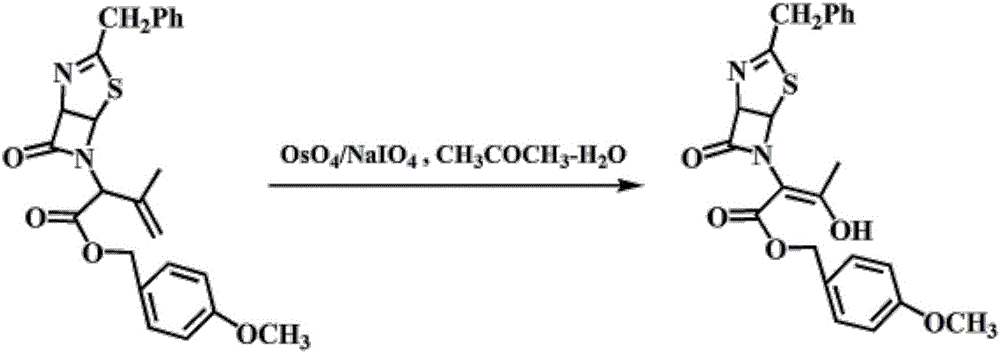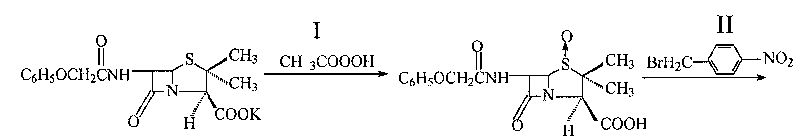Patents
Literature
30 results about "Penicillin G sulfoxide" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Method for preparing cephalosporin intermediate 7-ADCA
InactiveCN101735246AEasy to buy raw materialsHigh reaction yieldOrganic chemistryPyridiniumPenicillin G sulfoxide
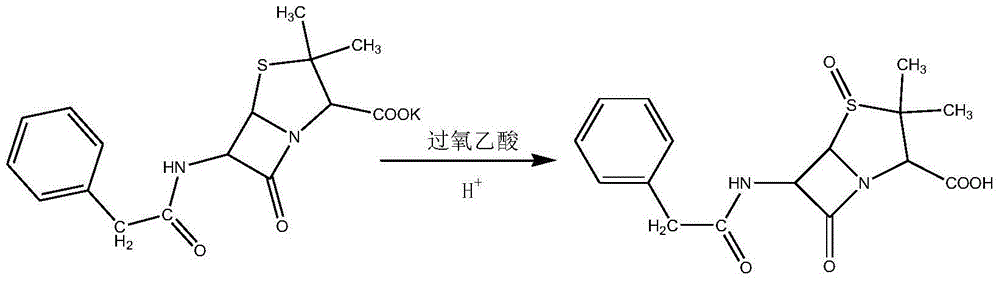
The invention discloses a method for preparing cephalosporin intermediate 7-ADCA. The preparation method has the advantages of high reaction yield and good quality. In the method, penicillin G sylvite is taken as a raw material and is oxidized into penicillin G sulfoxide by peracetic acid, wherein the mass yield reaches over 85 percent; hydrobromic acid-pyridinium is taken as a catalyst and bis(trimethylsily) urea is taken as a carboxyl protective reagent, and cephalosporin G acid is obtained through ring expansion and rearrangement, wherein the mass yield of a coarse product reaches 90 percent; and side chains are catalyzed and hydrolyzed by immobilized penicillin G acylase to prepare the 7-ADCA. On the basis of the penicillin G sylvite, the total mass yield reaches over 40 percent.
Owner:杨石
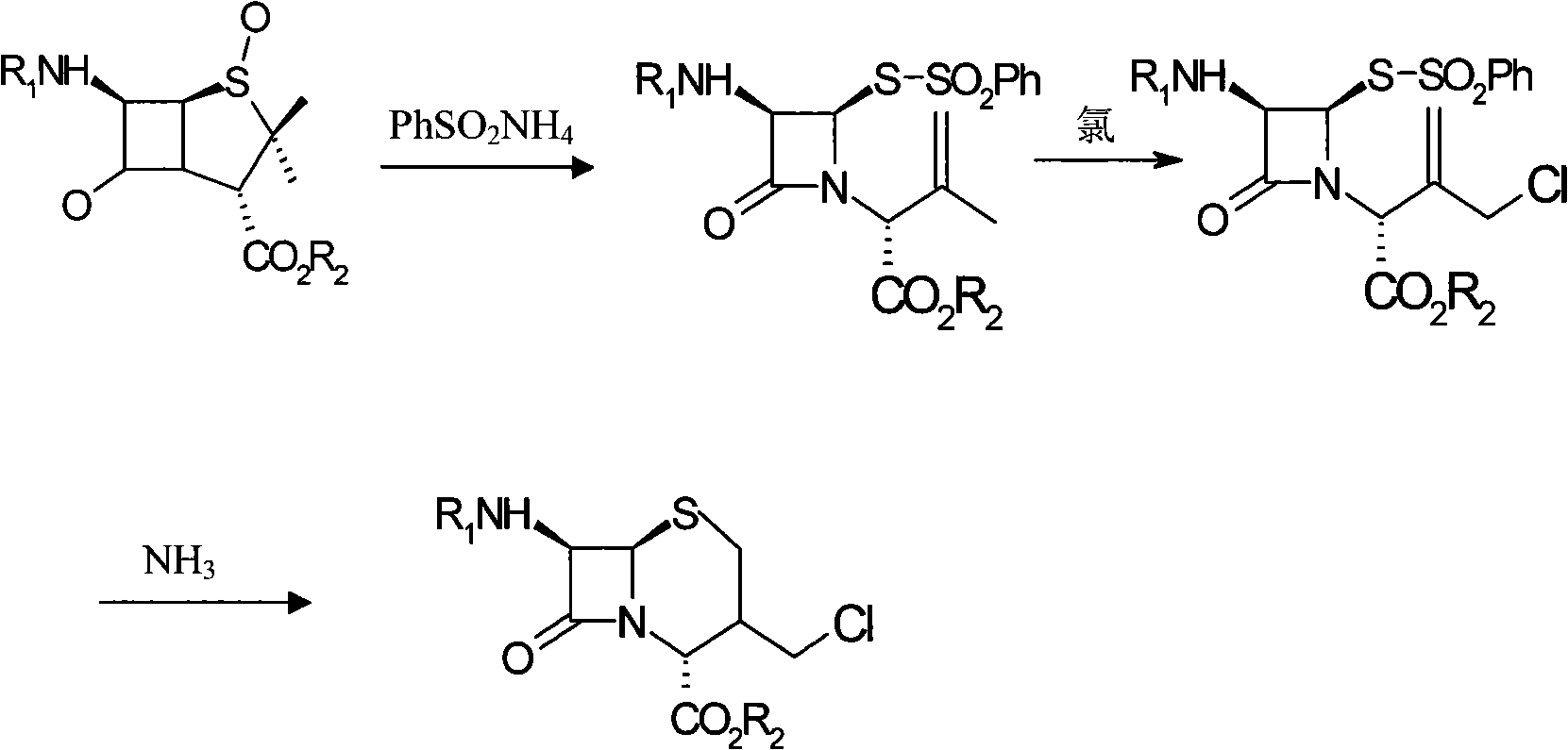
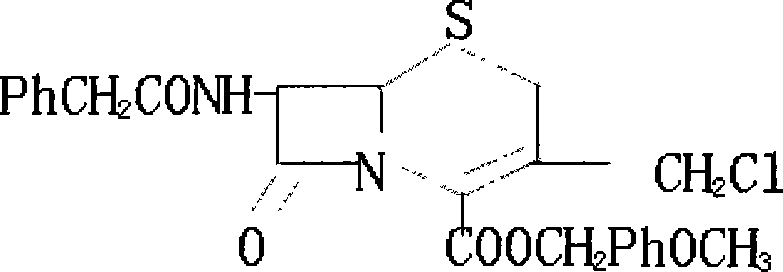
Method for synthesizing 7-phenylacetylamino-3-chloromethyl cephalosporin alkyl acid p-methoxybenzyl ester
InactiveCN101525340AFew reaction stepsSimple and fast operationOrganic chemistryPenicillinThiosulfinate
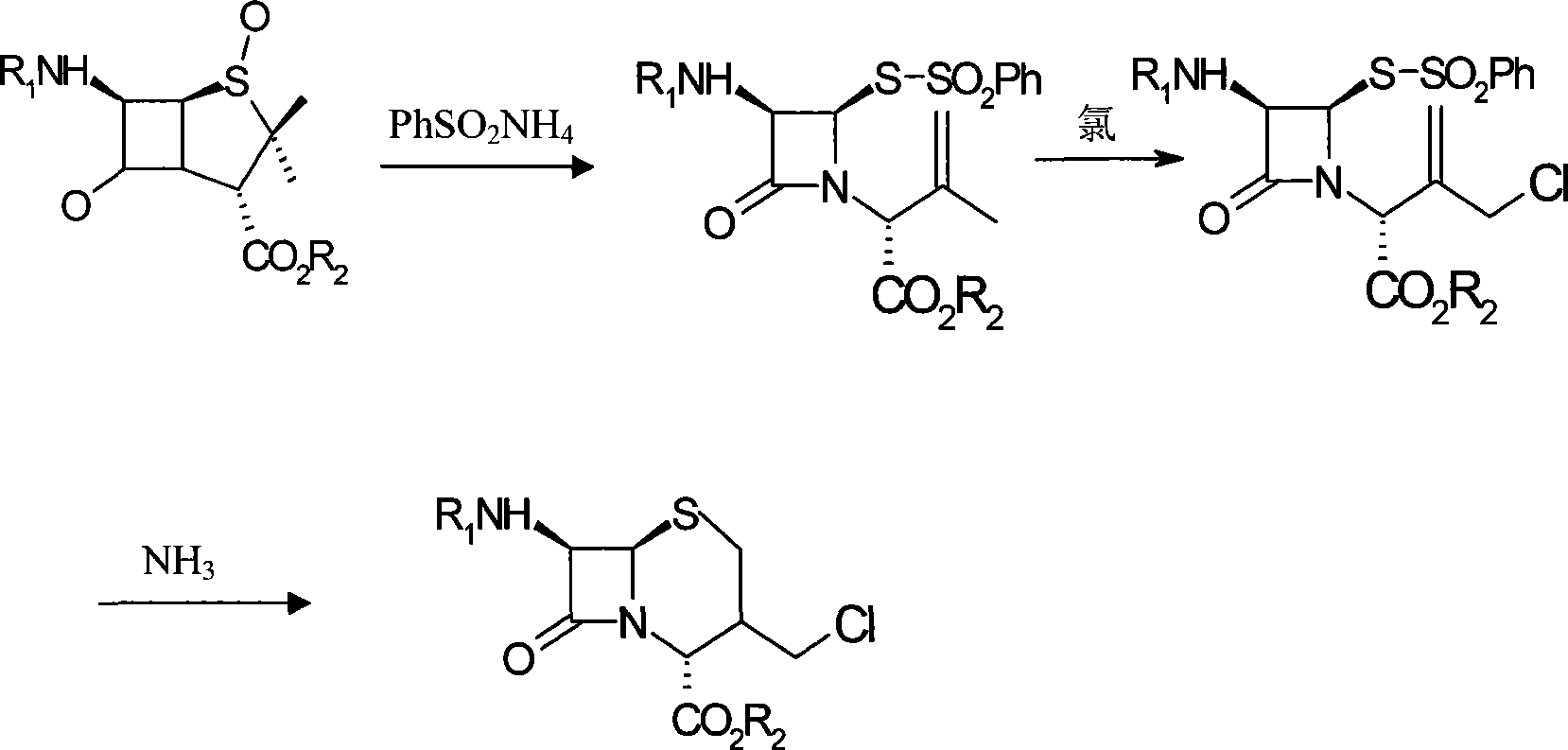
The invention belongs to the field of cephalosporin antibiotic medicaments, and in particular relates to a method for synthesizing 7-phenylacetylamino-3-chloromethyl cephalosporin alkyl acid p-methoxybenzyl ester (GCLE). The technical proposal is that the method comprises the following steps: reacting penicillin sulfoxide ester with ammonium benzene sulfinate and 2-mercaptobenzothiazole in dichloromethane, and steaming out a solvent at normal pressure to generate aza-cyclobutanone thiosulfinate intermediate; adding dichloromethane to the intermediate after cooling, stirring and introducing saturated brine ice for cooling, adding trichloro isocyanic acid for reaction so as to generate an allylic chlorination product of the aza-cyclobutanone thiosulfinate; and reducing the pressure and drying the allylic chlorination product by distillation, adding dimethyl formamide to the product, stirring and introducing saturated brine ice for cooling, adding ammonia for reaction, adding water and dichloromethane to the mixture, mixing and stirring the mixture, standing for layering, transferring a dichloromethane layer at the bottom layer to another reactor, and steaming out the solvent at the normal pressure to obtain a dry product which is the GCLE. The method has the advantages of mild reaction condition, few reaction steps, simple operation, short production cycle, and improved production efficiency.
Owner:SHANDONG FANGXING SCI & TECH DEV
Method for preparing penicillin-G-1-(S)-oxide
InactiveCN101974017AUniform particle size distributionNo coalescenceOrganic chemistryPenicillin G sulfoxideWater resources
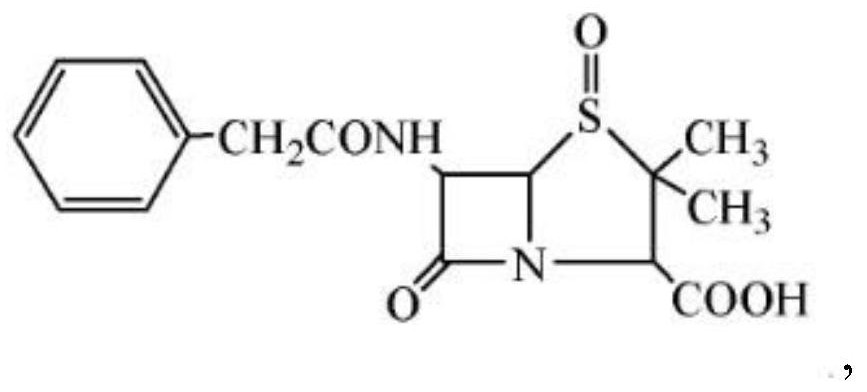
The invention relates to a method for preparing penicillin-G-1-(S)-oxide. In the method, penicillin G is used as a raw material, and the oxidization of the penicillin G and the crystallization of penicillin-G-1-(S)-oxide which is the oxidization product of the penicillin G are performed at the same time. A penicillin-G-1-(S)-oxide crystal product is obtained by directly adding an oxidant into penicillin G organic phase solution, reacting and crystallizing, the oxidization reaction and the crystallization are performed at the same time, and the crystal product directly precipitates in organic solution. Compared with the conventional direct oxidization process, the method has the advantages that: the operation flow is simplified considerably; the operation is simple and convenient; the labor intensity is lowered; and the production period is shortened obviously. The process of extracting the penicillin G from the organic phase back to a water phase and the process of regulating pH value with diluted acid and crystallizing are saved, so the consumption of waster resources is reduced greatly, the discharge of waste acid liquor is reduced, and the problem of environmental pollution is alleviated obviously. The high-performance liquid chromatography (HPLC) content of the product reaches over 99.0 percent, and the weight yield of products with a main particle size over 30 mu m is over 90 percent.
Owner:TIANJIN UNIV
Method for synthesizing 7-phenylacetamide-3-chloromethyl-4-cephalosporanic acid p-methoxybenzyl ester
InactiveCN101260116AHigh yieldEasy to operateOrganic chemistryPenicillin G sulfoxideSynthesis methods
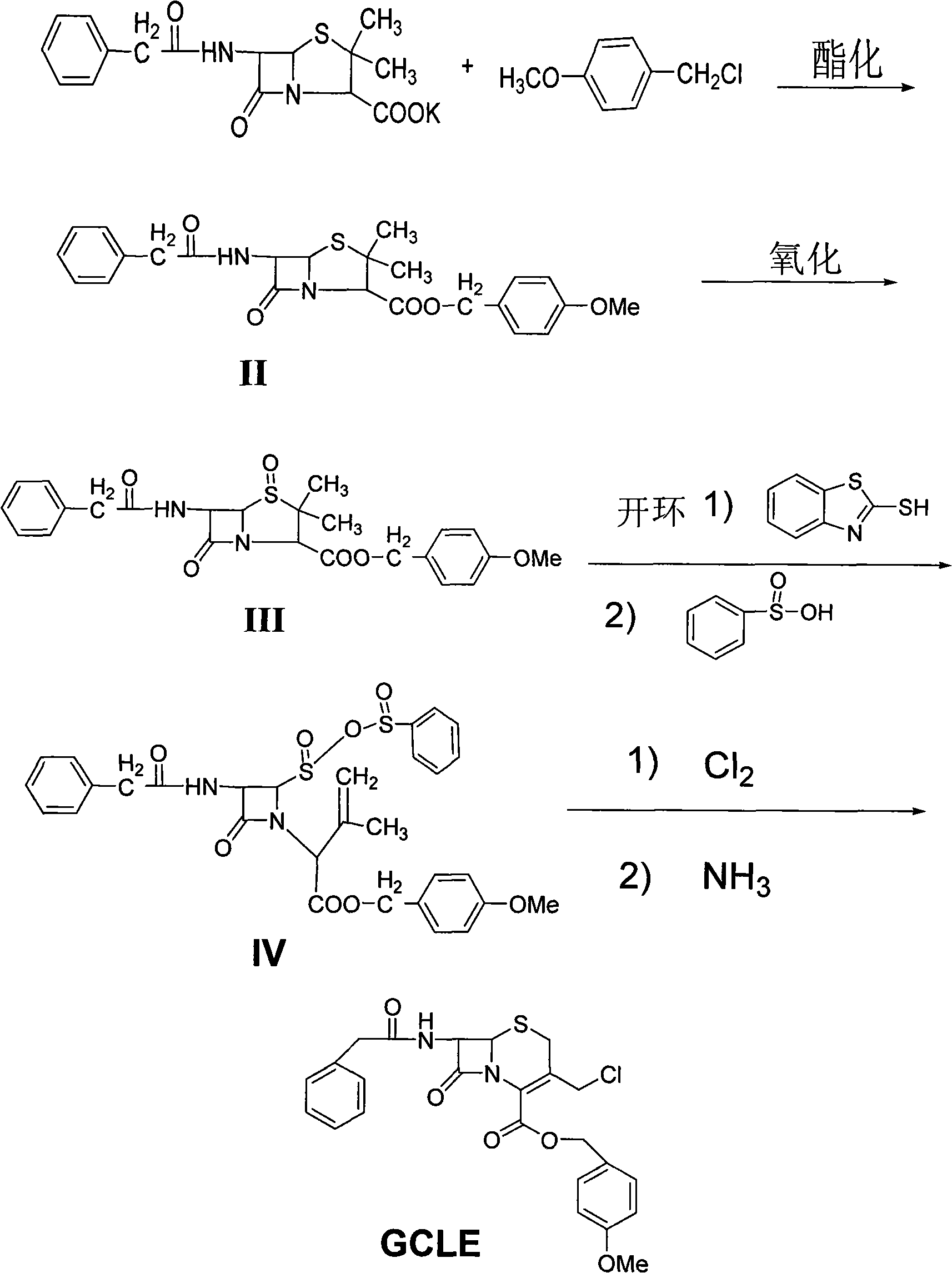
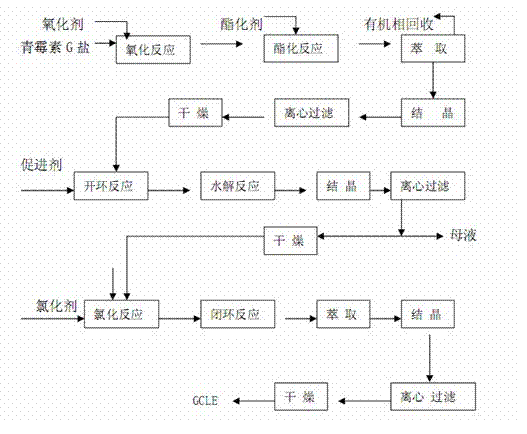
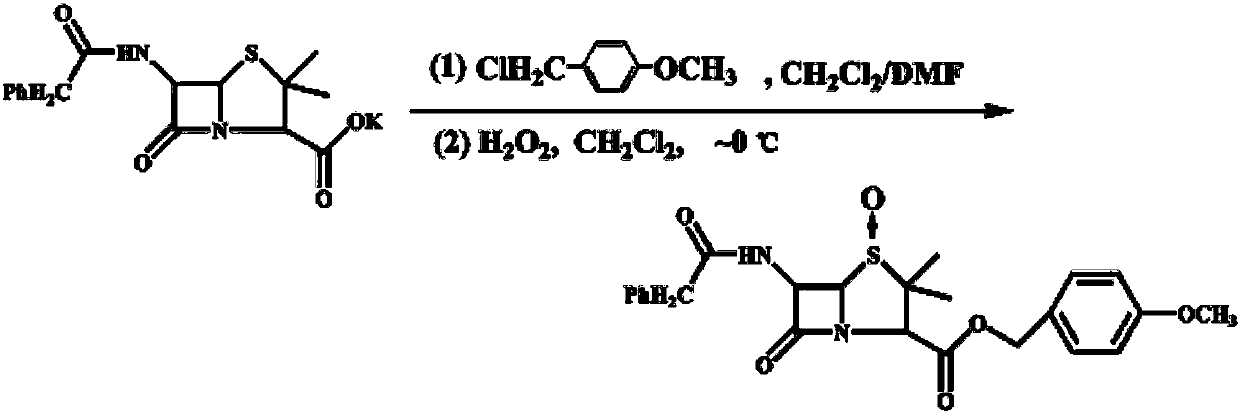
The invention discloses a synthesis method of 7- phenylacetamide-3-chloromethyl-4- cephalsporanic acid p- methoxybenzyl ester(GCLE). The invention takes the penicillin G kali salts mass made in China as starting materials; firstly, the penicillin G kali salts reacts with the p- methoxy benzyl chloride to obtain p- methoxybenzyl ester; secondly, the p- methoxybenzyl ester is oxidized by peroxy acetic acid to obtain penicillin sulfoxide ester; the penicillin sulfoxide ester firstly reacts with the 2- mercaptobenzothiazole, and then reacts with benzene sulfinic acid to obtain ring opening products; the ring opening products reacts with chlorine gases to obtain chlorination products; the chlorination products reacts with ammonia gases to obtain 7- phenylacetamide-3-chloromethyl-4- cephalsporanic acid p- methoxybenzyl ester(GCLE).
Owner:HUNAN NANXIN PHARMA CO LTD
Method for synthesizing penicillin G sulfoxide by using continuous flow reactor
ActiveCN111233892AAvoid security issuesEliminate the risk of contaminationOrganic chemistryChemical/physical/physico-chemical processesAcetic acidPenicillin G sulfoxide
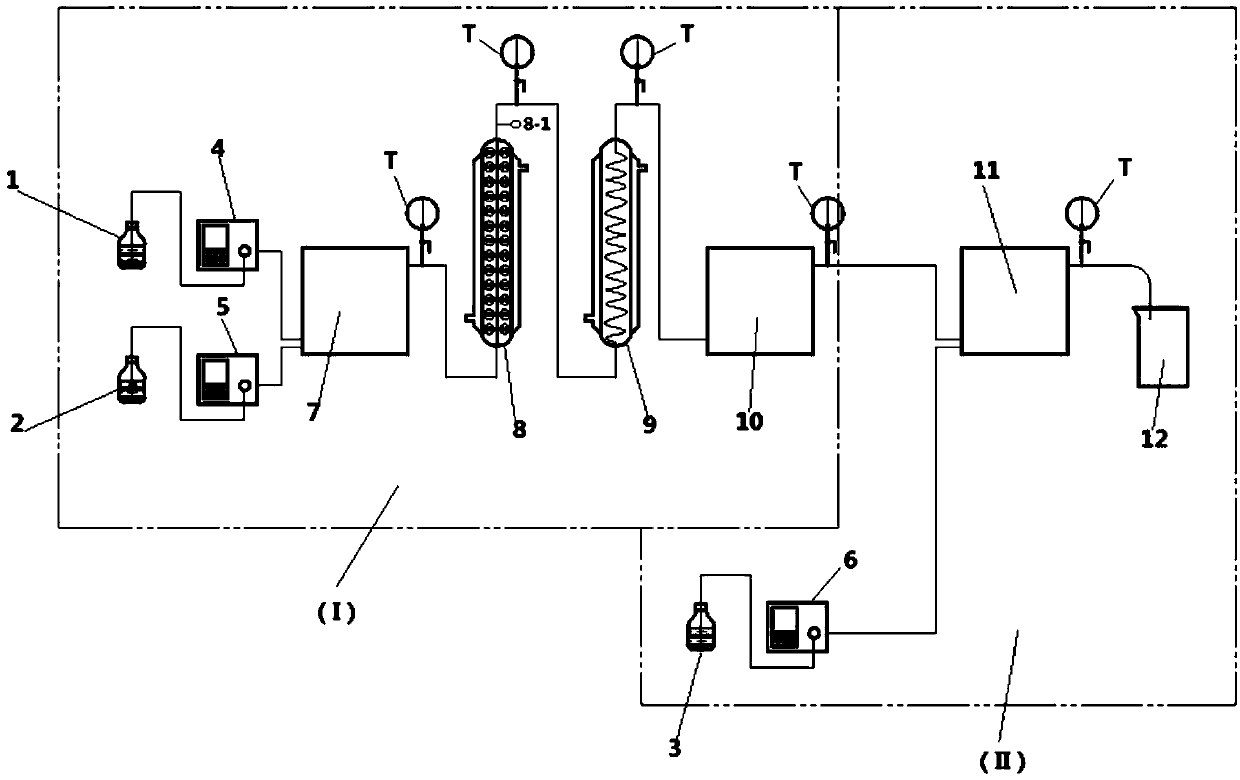
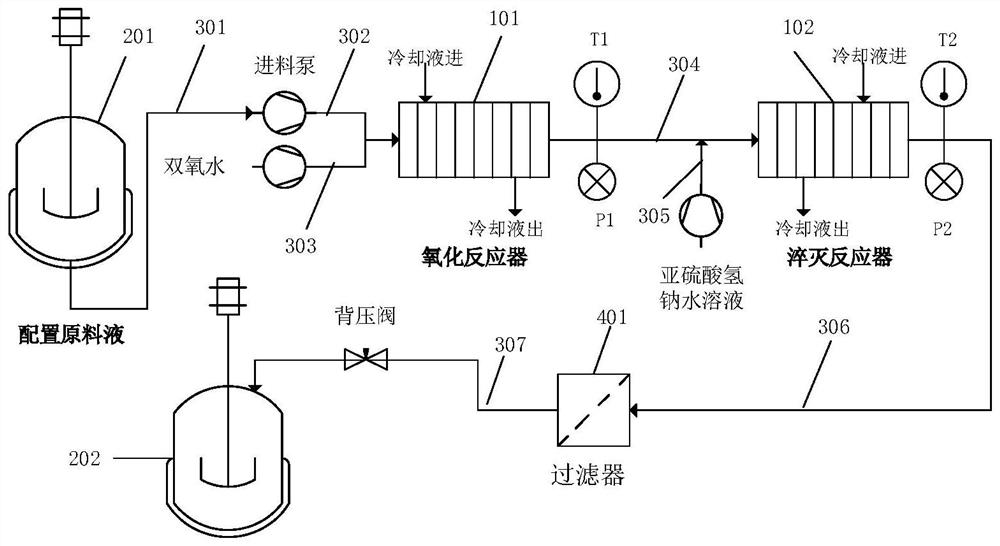
The invention provides a method for synthesizing penicillin G sulfoxide by using a continuous flow reactor, and the method comprises the following steps: (1) providing the continuous flow reactor which comprises a peroxidation reaction system and an oxidation reaction system; (2) respectively putting hydrogen peroxide and glacial acetic acid into a first containing bottle and a second containing bottle in the peroxidation reaction system, and enabling the hydrogen peroxide to be in contact with the glacial acetic acid so as to obtain peracetic acid; and (3) enabling the peracetic acid to enterthe oxidation reaction system through a pre-cooling module, and contacting the peracetic acid with a penicillin G potassium salt aqueous solution so as to obtain the penicillin G sulfoxide and a penicillin G sulfoxide aqueous solution. The method can be used for effectively synthesizing the penicillin G sulfoxide, and in the whole process of the method, reaction materials are easy to obtain, treatment after reaction is simple, industrial three wastes are easy to treat, the yield of a target product is high, and the method is very suitable for industrial production.
Owner:江苏悦新药业有限公司
Penicillin G sulfoxide composite crystal and preparation method thereof
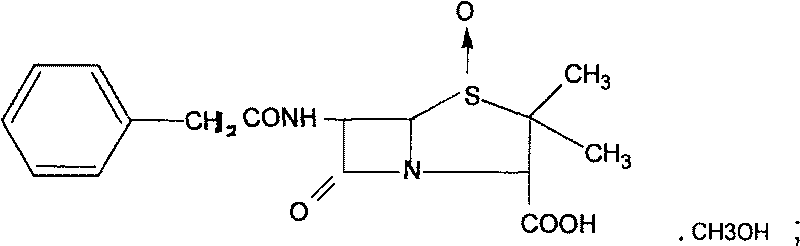
The invention discloses a novel composite crystal of penicillin G sulfoxide and preparation thereof. The composite crystal has a melting point of 106.9 DEG C., and percentage content of elements are C:53.35%, N:7.23%, O:24.9%, H:5.78%, S:8.70%. The preparation includes steps of: (a) dissolving the penicillin G sulfoxide in a methanol solution, or a solution containing methanol, or a organic solution containing methanol; (b) cooling down the solution containing penicillin G sulfoxide and methanol, turns penicillin G sulfoxide and methanol into saturated state or hypersaturated state gradually, thus crystals are separated. The inventive composite crystal is good in stability, and enhance yield and concentration of cethalosporanic acid effectively in ring-expanding reaction.
Owner:NORTH CHINA PHARMA COMPANY
Preparation method of penicillin G sulfoxide
ActiveCN102964355ASimple methodShort processing cycleOrganic chemistryPenicillin G sulfoxideFermentation
The invention discloses a novel method for preparing penicillin G sulfoxide, which comprises the following steps: a, selecting a 60000-120000 mu / g penicillin G fermentation solution, and dropwisely adding peroxyacetic acid to obtain a solution I; b, filtering the solution I to obtain a filtrate, and concentrating the filtrate through a nanofiltration membrane of which the molecular weight cut-off is 200-800Da, thus obtaining a concentrated solution; c, regulating the pH value of the concentrated solution with sulfuric acid, and filtering to obtain a penicillin G sulfoxide crude product; and d, dissolving the penicillin G sulfoxide crude product in a methanol water solution, recrystallizing, then cooling to 0-10 DEG C, filtering, washing the obtained crystals with methanol, swabbing off, and performing microwave drying on the obtained product to obtain the penicillin G sulfoxide. According to the invention, the method is simple, the process period is short, and extraction treatment can be performed without using a large amount of organic solvent. Thus, the method disclosed by the invention is low in cost and environment-friendly.
Owner:NORTH CHINA PHARMA COMPANY
Method for preparing thiazoline enol ester
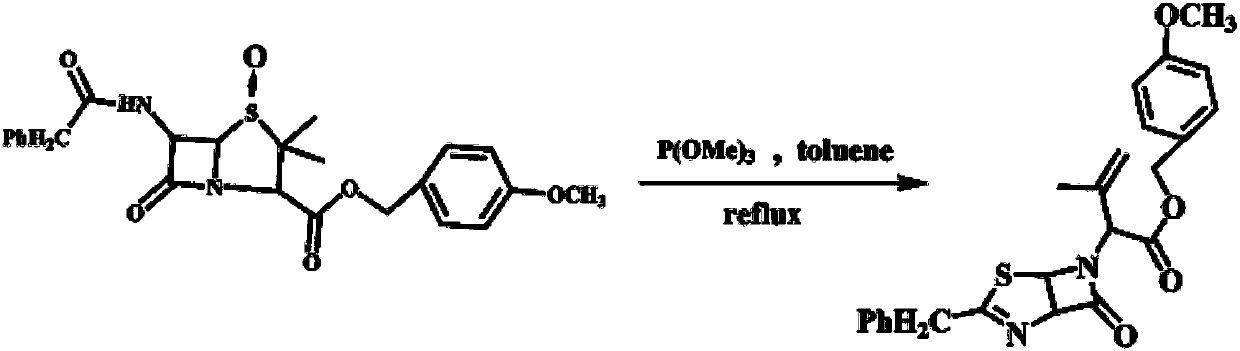
The invention discloses a method for preparing thiazoline enol ester, and belongs to the field of synthesis of compounds. Penicillin G sylvite is adopted as a raw material, and the method comprises the following steps that 1, penicillin G sylvite is adopted as the raw material, esterification and oxidizing are carried out, and penicillin sulfoxide ester is obtained; 2, penicillin sulfoxide ester is adopted as a raw material, open loop rearrangement occurs in a solvent, and a thiazoline-aza-ketone derivative with double bonds at the tail end is obtained; 3 the thiazoline-aza-ketone derivative with double bonds at the tail end is adopted as a raw material, oxidizing is carried out, and thiazoline enol ester is obtained. The method is easy to operate, low in cost, good in product quality, high in yield and suitable for industrial production, and the total yield reaches 82%.
Owner:浙江沙星科技股份有限公司
Method for preparing cephalosporin midbody penicillin sulfoxide
ActiveCN103467491AProcess loss is smallHigh product yieldOrganic chemistrySocial benefitsPenicillin G sulfoxide
The invention relates to a method for preparing cephalosporin midbody penicillin sulfoxide. According to the method for preparing the cephalosporin midbody penicillin sulfoxide, treatment such as extraction, decoloration, concentration and column chromatography is conducted on a penicillin fermentation extracting solution, and then the treated penicillin fermentation extracting solution is used as an initial raw material for preparation of the cephalosporin midbody penicillin sulfoxide. By the adoption of the method for preparing the cephalosporin midbody penicillin sulfoxide, the production steps are simplified, the production efficiency is improved, the energy consumption is reduced, emission is reduced, and remarkable economic benefits and remarkable social benefits are obtained.
Owner:ZHEJIANG ANGLIKANG PHARMA +1
Preparation method of cephalosporin antibiotic parent nucleus GCLE (7-phenylacetamido-3-cephem-4-carboxylic p-methoxybenzyl ester)
InactiveCN102863460AObvious beneficial effectHigh yieldOrganic chemistryN dimethylformamidePenicillin G sulfoxide
The invention discloses a preparation method of cephalosporin antibiotic parent nucleus GCLE (7-phenylacetamido-3-cephem-4-carboxylic p-methoxybenzyl ester), which comprises the following steps: oxidizing the raw material penicillin G potassium salt with peroxyacetic acid to obtain penicillin sulfoxide, reacting the penicillin sulfoxide with methoxybenzyl chloride to obtain penicillin sulfoxide ester, carrying out ring-opening reaction on the penicillin sulfoxide ester and a ring opening agent 2-mercaptobenzothiazole to prepare an intermediate azabutanone sulfinic acid under the protection of a protective agent; chlorinating the intermediate with a chlorinating agent, and cyclizing in a DMF (N,N-dimethylformamide) and nitrogenous environment to obtain the cephalosporin antibiotic parent nucleus GCLE. The invention optimizes the key technological conditions, fully utilizes the abundant penicillin industrial salt raw material resources in China, and successfully acquires a new technical scheme for preparing GCLE. The invention has the advantages of high safety, high efficiency, high yield and environmental protection, and provides important technical support for research and application of cephalosporin drugs.
Owner:广东省石油化工研究院
Production method of penicillin sulfoxide industrial products
The invention discloses a production method of penicillin sulfoxide industrial products, which belongs to the field of pharmaceutical chemicals. The method adopts the scheme that penicillin sulfoxideis suspended in a methanol or methanol aqueous solution, alkaline solution is added under the condition of stirring to dissolve penicillin sulfoxide, filtrate is obtained by filtering, an acid solution is added into the filtrate for crystallization, filtering and washing are carried out, and drying is performed to obtain a penicillin sulfoxide industrial product. The production method disclosed bythe invention is universally suitable for refining penicillin sulfoxide with various qualities prepared by various methods, the penicillin sulfoxide obtained by crystal transformation at normal temperature or low temperature is good in quality and high in yield, impurities are easy to separate and wash, the product is easy to dry, the stability is greatly improved, and the penicillin sulfoxide becomes a circulating commodity; in the obtained product, the penicillin sulfoxide content is more than or equal to 91.0 percent, the drying weight loss is less than or equal to 9.0 percent, the content(pure) is more than or equal to 99.9 percent, the light absorption (425nm) is less than or equal to 0.01, and the light transmission (425nm) is more than or equal to 90 percent.
Owner:NORTH CHINA PHARMA COMPANY
Method for preparing cephalosporin midbody penicillin sulfoxide
ActiveCN103467491BProcess loss is smallHigh product yieldOrganic chemistrySocial benefitsPenicillin G sulfoxide
The invention relates to a method for preparing cephalosporin midbody penicillin sulfoxide. According to the method for preparing the cephalosporin midbody penicillin sulfoxide, treatment such as extraction, decoloration, concentration and column chromatography is conducted on a penicillin fermentation extracting solution, and then the treated penicillin fermentation extracting solution is used as an initial raw material for preparation of the cephalosporin midbody penicillin sulfoxide. By the adoption of the method for preparing the cephalosporin midbody penicillin sulfoxide, the production steps are simplified, the production efficiency is improved, the energy consumption is reduced, emission is reduced, and remarkable economic benefits and remarkable social benefits are obtained.
Owner:ZHEJIANG ANGLIKANG PHARMA +1
Preparation method of penicillin G sulfoxide
ActiveCN102964355BEmission reductionIncrease friendlinessOrganic chemistryPenicillin G sulfoxideFermentation
The invention discloses a novel method for preparing penicillin G sulfoxide, which comprises the following steps: a, selecting a 60000-120000 mu / g penicillin G fermentation solution, and dropwisely adding peroxyacetic acid to obtain a solution I; b, filtering the solution I to obtain a filtrate, and concentrating the filtrate through a nanofiltration membrane of which the molecular weight cut-off is 200-800Da, thus obtaining a concentrated solution; c, regulating the pH value of the concentrated solution with sulfuric acid, and filtering to obtain a penicillin G sulfoxide crude product; and d, dissolving the penicillin G sulfoxide crude product in a methanol water solution, recrystallizing, then cooling to 0-10 DEG C, filtering, washing the obtained crystals with methanol, swabbing off, and performing microwave drying on the obtained product to obtain the penicillin G sulfoxide. According to the invention, the method is simple, the process period is short, and extraction treatment can be performed without using a large amount of organic solvent. Thus, the method disclosed by the invention is low in cost and environment-friendly.
Owner:NORTH CHINA PHARMA COMPANY
Method for synthesizing 7-phenylacetylaminodeacetoxycephalo G acid
InactiveCN102617602AReduce dosageLow cost of industrializationOrganic chemistryBenzenePenicillin G sulfoxide
The invention discloses a method for synthesizing 7-phenylacetylaminodeacetoxycephalo G acid. The method comprises the following step of: undergoing an esterification reaction on penicillin G sulfoxide in the presence of a trimethylhalosilane organic alkali to obtain a synthetic 7-phenylacetylaminodeacetoxycephalo G acid. The method is easy to operate, and is low in production cost.
Owner:YANAN BICON PHARMACEUTICAL LISTED COMPANY
Method for preparing penicillin sulfoxide ester through continuous flow
PendingCN114315862AImprove securityEasy to operateOrganic chemistryChemical recyclingPenicillin G sulfoxidePtru catalyst
The invention belongs to the technical field of chemistry and chemical engineering, and particularly relates to a method for preparing penicillin sulfoxide ester through continuous flow, which comprises the following steps: mixing hydrogen peroxide and a catalyst solution to prepare an oxidant, and carrying out continuous flow oxidation reaction on the oxidant and penicillin G potassium ester, and carrying out continuous flow quenching reaction on the oxidation reaction liquid by using a sodium hydrogen sulfite aqueous solution, and carrying out post-treatment to obtain the penicillin sulfoxide ester (GESO). According to the present invention, the method has advantages of simple operation, improved safety of the reaction unit, sustainability, high unit area production capacity compared with the traditional intermittent reaction mode, environmental protection and the like, the conversion rate of the preparation method can achieve 100%, the selectivity can achieve 99%, the separation yield can achieve 96%, and the method is suitable for industrial production. The content is greater than 99.5%, and the single impurity content index reaches the original research process standard.
Owner:SHENYANG RES INST OF CHEM IND
A kind of preparation method of thiazoline enol ester
The invention discloses a method for preparing thiazoline enol ester, and belongs to the field of synthesis of compounds. Penicillin G sylvite is adopted as a raw material, and the method comprises the following steps that 1, penicillin G sylvite is adopted as the raw material, esterification and oxidizing are carried out, and penicillin sulfoxide ester is obtained; 2, penicillin sulfoxide ester is adopted as a raw material, open loop rearrangement occurs in a solvent, and a thiazoline-aza-ketone derivative with double bonds at the tail end is obtained; 3 the thiazoline-aza-ketone derivative with double bonds at the tail end is adopted as a raw material, oxidizing is carried out, and thiazoline enol ester is obtained. The method is easy to operate, low in cost, good in product quality, high in yield and suitable for industrial production, and the total yield reaches 82%.
Owner:SHAXING CHEM TAIZHOU CITY
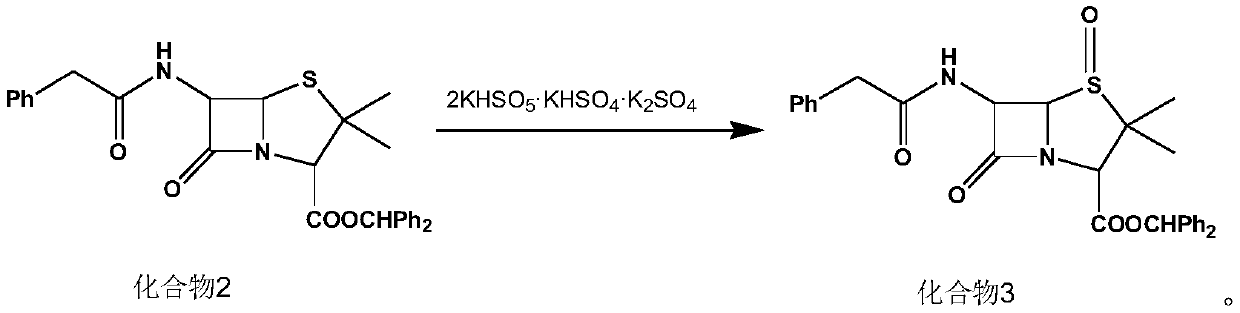
A kind of synthetic method of penicillin g sulfoxide benzhydryl ester
ActiveCN110845516BSolve pollutionRaw materials are easy to getOrganic chemistryPenicillin G sulfoxideMedicinal chemistry
The invention discloses a synthesis method of penicillin G sulfoxide benzhydryl ester. Using penicillin G potassium salt as the starting material, first esterification, and then 2KHSO 5 ·KHSO 4 ·K 2 SO 4 The oxidative synthesis of penicillin G sulfoxide benzhydryl ester has a product purity of more than 99% and a total molar yield of more than 95%, and is suitable for industrial production. The invention has the advantages of readily available raw materials, high yield, less pollution, high safety factor, easy operation and the like.
Owner:YANCHENG KAIYUAN MEDICINE CHEM
Method for synthesizing 7-phenylacetylamino-3-chloromethyl cephalosporin alkyl acid p-methoxybenzyl ester
InactiveCN101525340BFew reaction stepsSimple and fast operationOrganic chemistryPenicillinThiosulfinate
The invention belongs to the field of cephalosporin antibiotic medicaments, and in particular relates to a method for synthesizing 7-phenylacetylamino-3-chloromethyl cephalosporin alkyl acid p-methoxybenzyl ester (GCLE). The technical proposal is that the method comprises the following steps: reacting penicillin sulfoxide ester with ammonium benzene sulfinate and 2-mercaptobenzothiazole in dichloromethane, and steaming out a solvent at normal pressure to generate aza-cyclobutanone thiosulfinate intermediate; adding dichloromethane to the intermediate after cooling, stirring and introducing saturated brine ice for cooling, adding trichloro isocyanic acid for reaction so as to generate an allylic chlorination product of the aza-cyclobutanone thiosulfinate; and reducing the pressure and drying the allylic chlorination product by distillation, adding dimethyl formamide to the product, stirring and introducing saturated brine ice for cooling, adding ammonia for reaction, adding water and dichloromethane to the mixture, mixing and stirring the mixture, standing for layering, transferring a dichloromethane layer at the bottom layer to another reactor, and steaming out the solvent at the normal pressure to obtain a dry product which is the GCLE. The method has the advantages of mild reaction condition, few reaction steps, simple operation, short production cycle, and improved production efficiency.
Owner:SHANDONG FANGXING SCI & TECH DEV
A kind of extraction recovery method of penicillin sulfoxide
ActiveCN105418640BRealize zero pollutionQuality improvementAntibacterial agentsOrganic active ingredientsUpper urinary tract infectionPenicillin G sulfoxide
Owner:NORTH CHINA PHARMA COMPANY +1
Penicillin G sulfoxide dimethyl formamide (DMF) composite crystal and preparation method thereof
ActiveCN103059044BImprove stabilityHigh efficiency of ring expansion rearrangement reactionOrganic chemistryCelsius DegreePenicillin G sulfoxide
The invention discloses a penicillin G sulfoxide dimethyl formamide (DMF) composite crystal and a preparation method thereof. The melting point of the composite crystal is not lower than 101 Celsius degrees. The element contents are that 53.6% of carbon (C), 9.9% of nitrogen (N), 22.8% of oxygen (O), 5.9% of hydrogen (H) and 7.55% of sulfur (S). The preparation method is that firstly, the penicillin G sulfoxide is dissolved in the DMF water solution, the water solution containing the DMF or organic solution containing the DMF to obtain the DMF mixed solution of the penicillin G sulfoxide, and then the DMF mixed solution of the penicillin G sulfoxide is cooled and placed quietly, the penicillin G sulfoxide DMF composite crystal is separated out, and the penicillin G sulfoxide DMF composite crystal is filtered, washed and dried. The penicillin G sulfoxide DMF composite crystal is good in stability, low in water content and does not contain impurities to damage protection for the esterification protective reaction. The process of the esterification protective reaction and a ring-enlargement rearrangement reaction is ensured in process of producing cephalosporin alkyl acid. The cephalosporin alkyl acid obtained from the ring-enlargement rearrangement reaction is high in yield and good in quality.
Owner:NORTH CHINA PHARMA COMPANY
Method for preparing 7-phenylacetylamino-3-methylcephalosporanic acid
The invention discloses a method for preparing 7-phenylacetylamino-3-methylcephalosporanic acid. The method comprises the following steps of: a, dissolving penicillin G sulfoxide into organic solution, and adding an esterifying agent into the solution to perform esterification reaction; b, adding a catalyst into the solution to perform rearrangement ring-enlarging reaction, hydrolysis and alkali dissolution; c, standing the rearrangement ring-enlarging reaction solution and splitting phases; d, adding an organic solvent containing quaternary ammonium salt cationic surfactant into brine solution, stirring the solution uniformly, standing the solution and splitting the phases; and e, after the aqueous phase is subjected to degassing treatment, directly performing a cracking process to obtain the 7-phenylacetylamino-3-methylcephalosporanic acid, and returning the organic solution to the process d for recycle. The method greatly improves the purity of CEFG, and effectively avoids a process for crystallizing the CEFG in the traditional process so as to simplify the process for preparing the CEFG, reduce the production and manufacture costs, avoid wastewater discharge and reduce environmental pollution at the same time.
Owner:NORTH CHINA PHARMA COMPANY
Penicillin G sulfoxide dimethylacetamide (DMA) composite crystal and preparation method thereof
ActiveCN103030648BImprove stabilityEasy to storeCarboxylic acid amide separation/purificationPenicillin G sulfoxideAqueous solution
Owner:NORTH CHINA PHARMA COMPANY
Preparation of 7-phenylacetamide-3-chloromethyl cephalosporanic acid p-methoxybenzyl ester
ActiveCN107033162BReduce lossesEasy to operateOrganic chemistrySodium methoxidePenicillin G sulfoxide
The invention belongs to the field of synthesis of cephalosporin drug intermediates and disclosesa preparation method of 7-phenylacetamide-3-chloromethyl-cephalosporanic acid p-methoxybenzyl ester (short for GCLE). According to the method, penicillin G potassium salt taken as an initial raw material reacts with 4-methoxybenzylchloride firstly, penicillin sulfoxide ester is prepared through oxidation with peracetic acid and reacts with 2-mercaptobenzothiazole firstly, then a product reacts with benzene sulfinic acid, a ring opening product is obtained and reacts with chlorine, sodium methoxide is used for ring closing under the action of a methanol solvent, and 7-phenylacetamide-3-chloromethyl-cephalosporanic acid p-methoxybenzyl ester is obtained. The solvent is simple and easy to recover, reaction conditions are mild, operation is simple and convenient, and industrial production is easy.
Owner:湖北凌晟药业股份有限公司
Method for preparing p-nitrobenzyl-7-phenoxyacetamido-3-exomethylenecepham-4-carboxylate-1-beta-oxide
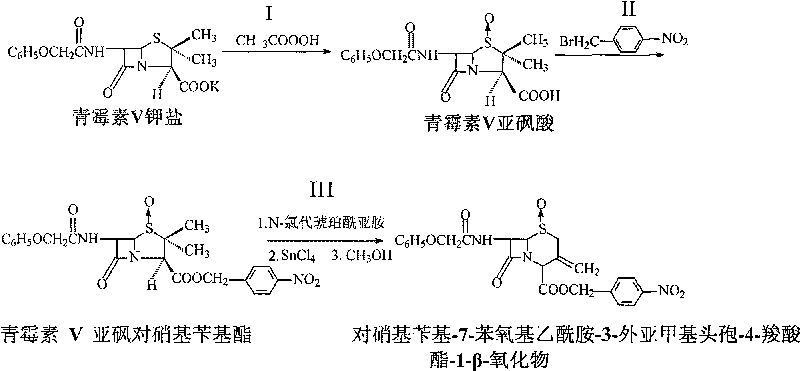
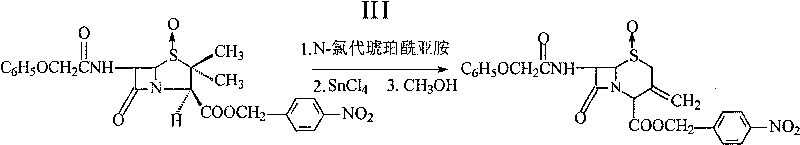
InactiveCN101190921BHigh reaction conversion rateHigh reaction yieldOrganic chemistryN-ChlorosuccinimideCarboxylic salt
The invention pertains to a novel preparation method of p-nitrobenzyl-7-phenoxy-ace-tamide-3-exomethylenecepham-4-carboxylate-1-Beta-oxide. Penicillin V potassium salt and peracetic acid react to obtain penicillin V sulfoxide by reverse titration; the penicillin V sulfoxide and p-monobromo-methylbenzeneare nitrate are treated with reflux reaction under the action of triethylamine in acetone solution to prepare penicillin V sulfoxide p-nitrobenzyl ester group; the penicillin V sulfoxide p-nitrobenzyl ester group under the action of N-chlorosuccinimide and calcium oxide opens five ring and closes hexatomic ring again under the action of stannic chloride and then reacts with methanol to prepare the p-nitrobenzyl-7-phenoxy-ace-tamide-3-exomethylenecepham-4-carboxylate- 1-Beta-oxide. The preparation method has the advantages of short preparation and reaction time and production cycles, low cost of raw materials, less dosage and high yield.
Owner:吉林省石油化工设计研究院
Penicillin G sulfoxide dimethylacetamide (DMA) composite crystal and preparation method thereof
ActiveCN103030648AImprove stabilityEasy to storeCarboxylic acid amide separation/purificationPenicillin G sulfoxideAqueous solution
The invention discloses a penicillin G sulfoxide dimethylacetamide (DMA) composite crystal and a preparation method thereof. The molecular formula of the composite crystal is C20H27N3O6S and the molecular weight of the composite crystal is 437.5. The pareparation method comprises the following steps: (a) dissolving penicillin G sulfoxide into a DMA solution or an aqueous solution containing the DMA or a mixed organic solution containing the DMA; (b) cooling the solution containing the penicillin G sulfoxide and the DMA so as to enable the penicillin G sulfoxide and the DMA to gradually reach a saturated or supersaturated state and separate out the crystal; and separating and drying the crystal to obtain the penicillin G sulfoxide DMA composite crystal. The composite crystal provided by the invention has high stability, does not contain impurities which damage esterification protective reaction, is extremely favorable for esterification protection reaction and ring expanding rearrangement reaction, increases the yield of the ring expanding rearrangement reaction, and improves the product quality.
Owner:NORTH CHINA PHARMA COMPANY
Industrial production method for preparing penicillin sulfoxide by continuously oxidizing penicillin
ActiveCN113214292AEasy to operateSimplify cumbersome operationsOrganic chemistryPenicillin G sulfoxideReaction temperature
The invention discloses an industrial production method for preparing penicillin sulfoxide by continuously oxidizing penicillin, and belongs to the field of pharmaceutical chemicals; a continuous reaction method is adopted, a penicillin solution and peracetic acid or other oxidants simultaneously flow into a mixer or a microreactor according to a certain proportion, and a penicillin sulfoxide solution is obtained by reaction at high temperature and in short time; the method can be used for an oxidation process of a process for preparing penicillin sulfoxide by taking penicillin fermentation liquor, filtrate, BA, RB, penicillin industrial salt and the like as initial raw materials, and solves the problem that the reaction temperature and the reaction time are influenced by mass transfer and low heat transfer efficiency of batch reaction; therefore, the problems of poor reaction liquid quality and low reaction yield are solved, and the yield of penicillin sulfoxide is high.
Owner:NORTH CHINA PHARMA COMPANY
Penicillin G sulfoxide dimethyl formamide (DMF) composite crystal and preparation method thereof
ActiveCN103059044AImprove stabilityHigh efficiency of ring expansion rearrangement reactionOrganic chemistryPenicillinCelsius Degree
The invention discloses a penicillin G sulfoxide dimethyl formamide (DMF) composite crystal and a preparation method thereof. The melting point of the composite crystal is not lower than 101 Celsius degrees. The element contents are that 53.6% of carbon (C), 9.9% of nitrogen (N), 22.8% of oxygen (O), 5.9% of hydrogen (H) and 7.55% of sulfur (S). The preparation method is that firstly, the penicillin G sulfoxide is dissolved in the DMF water solution, the water solution containing the DMF or organic solution containing the DMF to obtain the DMF mixed solution of the penicillin G sulfoxide, and then the DMF mixed solution of the penicillin G sulfoxide is cooled and placed quietly, the penicillin G sulfoxide DMF composite crystal is separated out, and the penicillin G sulfoxide DMF composite crystal is filtered, washed and dried. The penicillin G sulfoxide DMF composite crystal is good in stability, low in water content and does not contain impurities to damage protection for the esterification protective reaction. The process of the esterification protective reaction and a ring-enlargement rearrangement reaction is ensured in process of producing cephalosporin alkyl acid. The cephalosporin alkyl acid obtained from the ring-enlargement rearrangement reaction is high in yield and good in quality.
Owner:NORTH CHINA PHARMA COMPANY
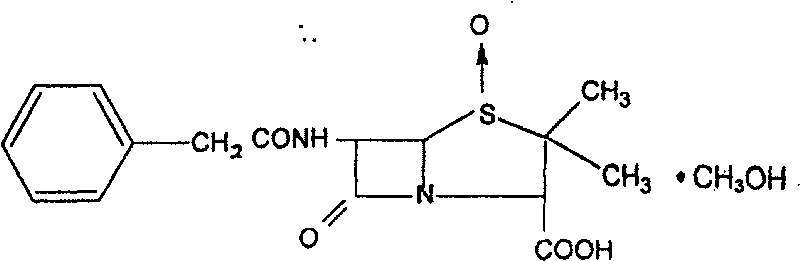
Penicillin G sulfoxide composite crystal and preparation method thereof
The invention discloses a novel composite crystal of penicillin G sulfoxide and preparation thereof. The composite crystal has a melting point of 106.9 DEG C., and percentage content of elements are C:53.35%, N:7.23%, O:24.9%, H:5.78%, S:8.70%. The preparation includes steps of: (a) dissolving the penicillin G sulfoxide in a methanol solution, or a solution containing methanol, or a organic solution containing methanol; (b) cooling down the solution containing penicillin G sulfoxide and methanol, turns penicillin G sulfoxide and methanol into saturated state or hypersaturated state gradually,thus crystals are separated. The inventive composite crystal is good in stability, and enhance yield and concentration of cethalosporanic acid effectively in ring-expanding reaction.
Owner:NORTH CHINA PHARMA COMPANY
Penicillin G sulfoxide diphenyl methyl ester synthesis method
ActiveCN110845516ASolve pollutionRaw materials are easy to getOrganic chemistryPenicillin G sulfoxideMedicinal chemistry
The invention discloses a penicillin G sulfoxide diphenyl methyl ester synthesis method, which comprises: esterifying a penicillin G potassium salt as an initial raw material, and oxidizing through 2KHSO5.KHSO4.K2SO4 to synthesize the penicillin G sulfoxide diphenyl methyl ester. According to the invention, the product purity is more than 99%, the total molar yield is more than 95%, the method issuitable for industrial production, and the method has advantages of readily available raw materials, high yield, less pollution, high safety factor, easy operation and the like.
Owner:YANCHENG KAIYUAN MEDICINE CHEM
Application of supported catalyst in continuous preparation of penicillin sulfoxide ester
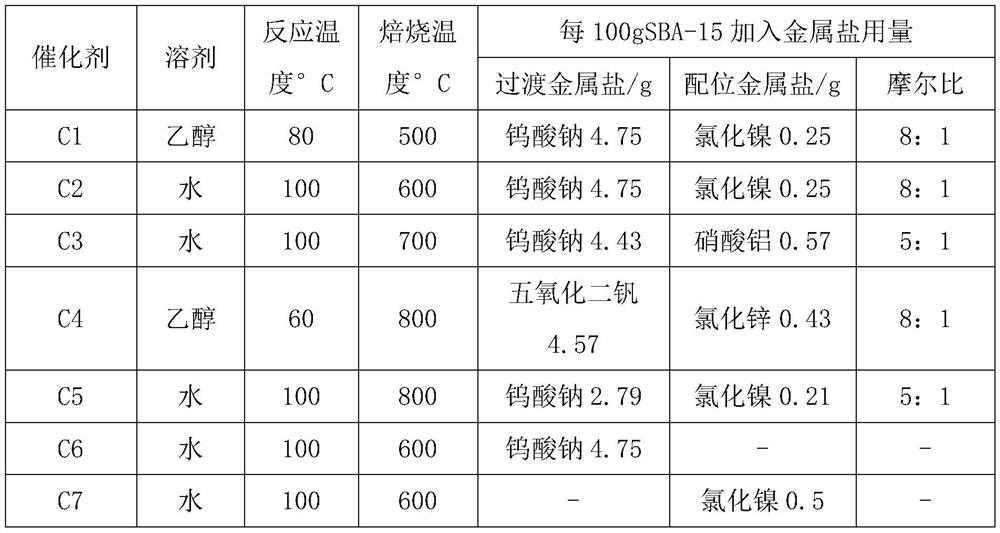
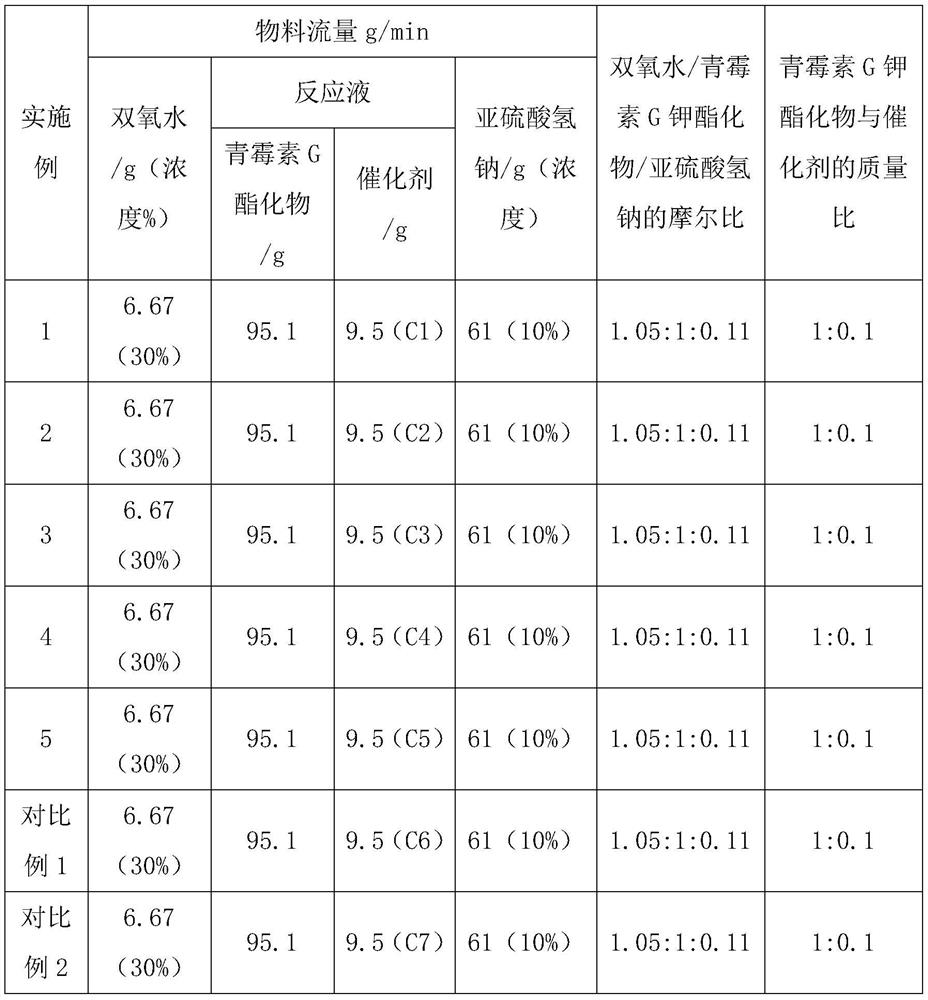
ActiveCN114437110AEasy to makeHigh catalytic efficiencyOrganic chemistryMolecular sieve catalystsPenicillin G sulfoxidePtru catalyst
The invention belongs to the technical field of chemistry and chemical engineering. The invention specifically relates to an application of a supported catalyst in continuous preparation of penicillin sulfoxide ester. A carrier of the supported catalyst is an ordered mesoporous material SBA-15, an active component of the supported catalyst is a metal salt, and the mass of the active component metal salt is 1-10% of the mass of the ordered mesoporous material SBA-15. Under the action of a supported catalyst, hydrogen peroxide and penicillin G potassium ester are subjected to a continuous flow oxidation reaction, an oxidation reaction solution is subjected to a continuous flow quenching reaction with a sodium hydrogen sulfite aqueous solution, and a quenching reaction solution is subjected to post-treatment to obtain the penicillin sulfoxide ester. The catalyst provided by the invention has the advantages of simple preparation, high catalytic efficiency, high selectivity and the like, and can be recycled after simple activation. By adopting the supported catalyst disclosed by the invention, the reaction conversion rate of penicillin sulfoxide ester preparation can reach 99.5%, the selectivity can reach 100%, the separation yield can reach 95.9%, and the content can reach 99.3%.
Owner:SHENYANG RES INST OF CHEM IND
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com