Penicillin G sulfoxide dimethyl formamide (DMF) composite crystal and preparation method thereof
A technology of composite crystal and penicillin, applied in the field of medicine, can solve the problems of affecting the yield of ring expansion rearrangement reaction, unstable methanol composite crystal, affecting esterification protection reaction, etc., and achieves high stability, simplified solvent system, moisture content and the like. reduced effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0042] At room temperature, put 30 grams of penicillin G sulfoxide (water content 15%) into a 100ml single-mouth eggplant-shaped bottle, add DMF12.8ml, and dissolve in an ultrasonic pool at 35°C to form a DMF mixed solution of penicillin G sulfoxide . Cool the single-mouth eggplant-shaped bottle, make the temperature of the mixed solution drop to -10°C and let it stand for 24 hours, filter to obtain a filter cake, wash the filter cake with 80ml of petroleum ether, and dry to obtain 26.6 grams of penicillin G sulfoxide DMF complex crystals (molar yield 86.3%).
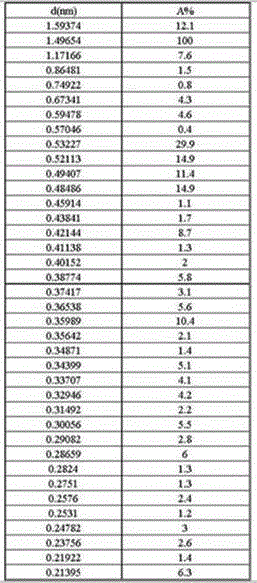
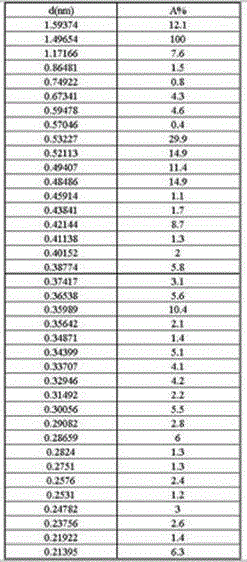
[0043] The X-ray powder diffraction spectrum of the penicillin G sulfoxide DMF composite crystal that embodiment 1 obtains, as shown below, described X-ray powder diffraction spectrum is to pass the monochromator silk filter to obtain by the copper ray of λ=1.54059 angstrom .
[0044]
[0045] where d is the interplanar spacing, and A% is the relative intensity.
[0046] The melting point of the penicillin G sulfo...
Embodiment 2
[0048] At room temperature, put 30 grams of penicillin G sulfoxide (0.3% water content) into a 100ml one-mouth eggplant-shaped bottle, add 30ml of DMF (that is, N,N-dimethylformamide), and carry out in an ultrasonic pool at 30°C Dissolved to obtain a DMF mixed solution of penicillin G sulfoxide. Cool the single-mouth eggplant-shaped bottle, lower the temperature of the mixed solution to -20°C and let it stand for 24 hours, filter to obtain a filter cake, wash the filter cake with 100ml of petroleum ether, and dry to obtain 20.5 grams of penicillin G sulfoxide DMF complex crystals (molar yield 56.6 %), melting point is the heat flow curve measured by differential scanning calorimeter.
[0049] The melting point of the penicillin G sulfoxide complex crystal obtained in Example 2 was measured by differential scanning calorimetry: 101.0°C.
Embodiment 3
[0051] At room temperature, put 25 grams of penicillin G sulfoxide (water content 0.2%) into a 250ml one-mouth eggplant-shaped bottle, add 100ml of the DMF (N,N-dimethylformamide) mother solution in Example 2, and at 40°C, Dissolve in an ultrasonic bath to form a mixed solution of penicillin G sulfoxide in DMF (N,N-dimethylformamide). This one-mouth eggplant-shaped bottle was cooled, the temperature of the mixed solution was lowered to 5° C. and allowed to stand for 24 hours, filtered to obtain a filter cake, which was washed with 80 ml of petroleum ether, and dried to obtain 20.3 grams of penicillin G sulfoxide DMF complex crystals (molar yield 68.5%).
[0052] The melting point of the penicillin G sulfoxide complex crystal obtained in Example 3 was measured by differential scanning calorimetry: 101.6°C.
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com