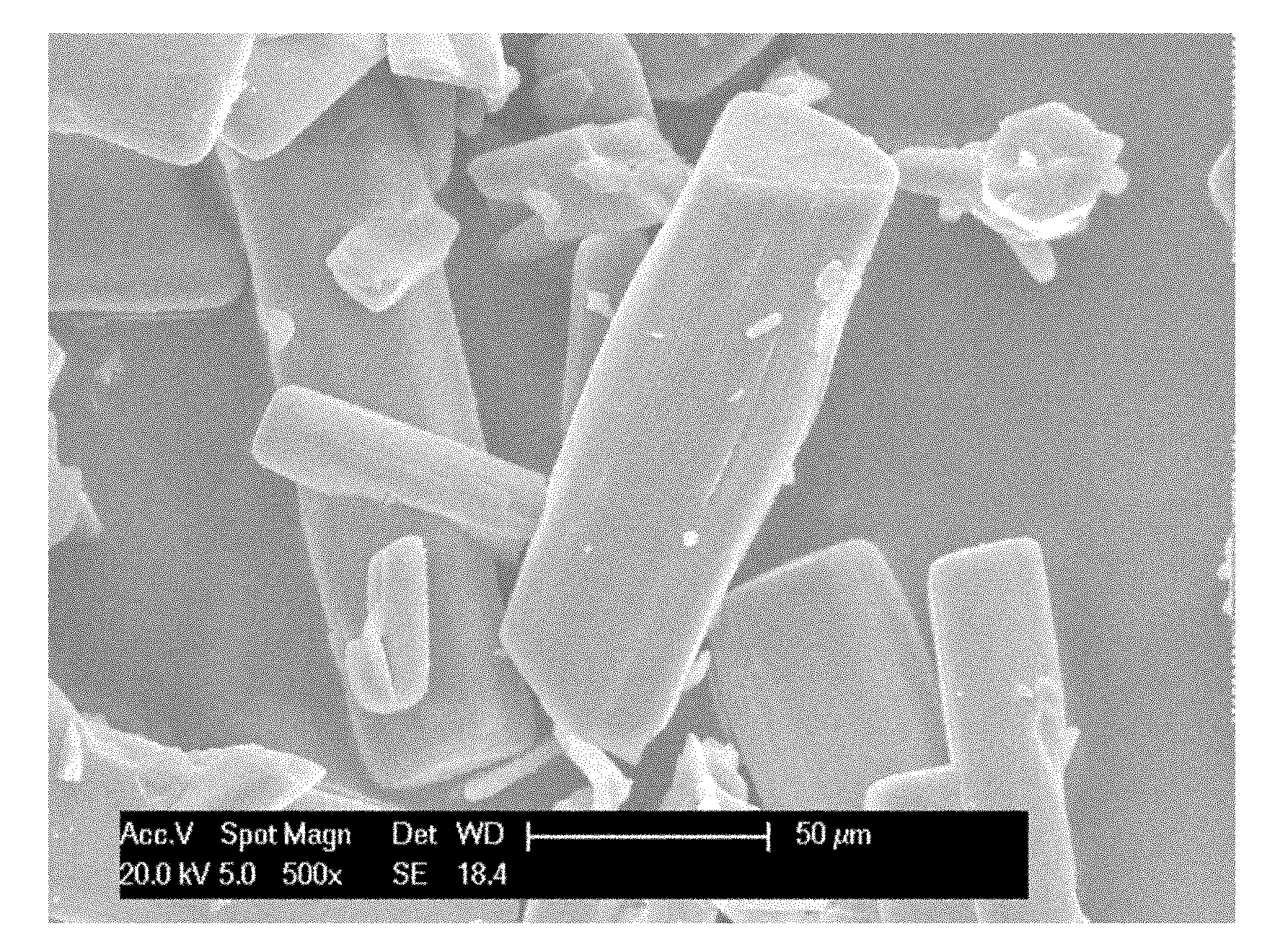
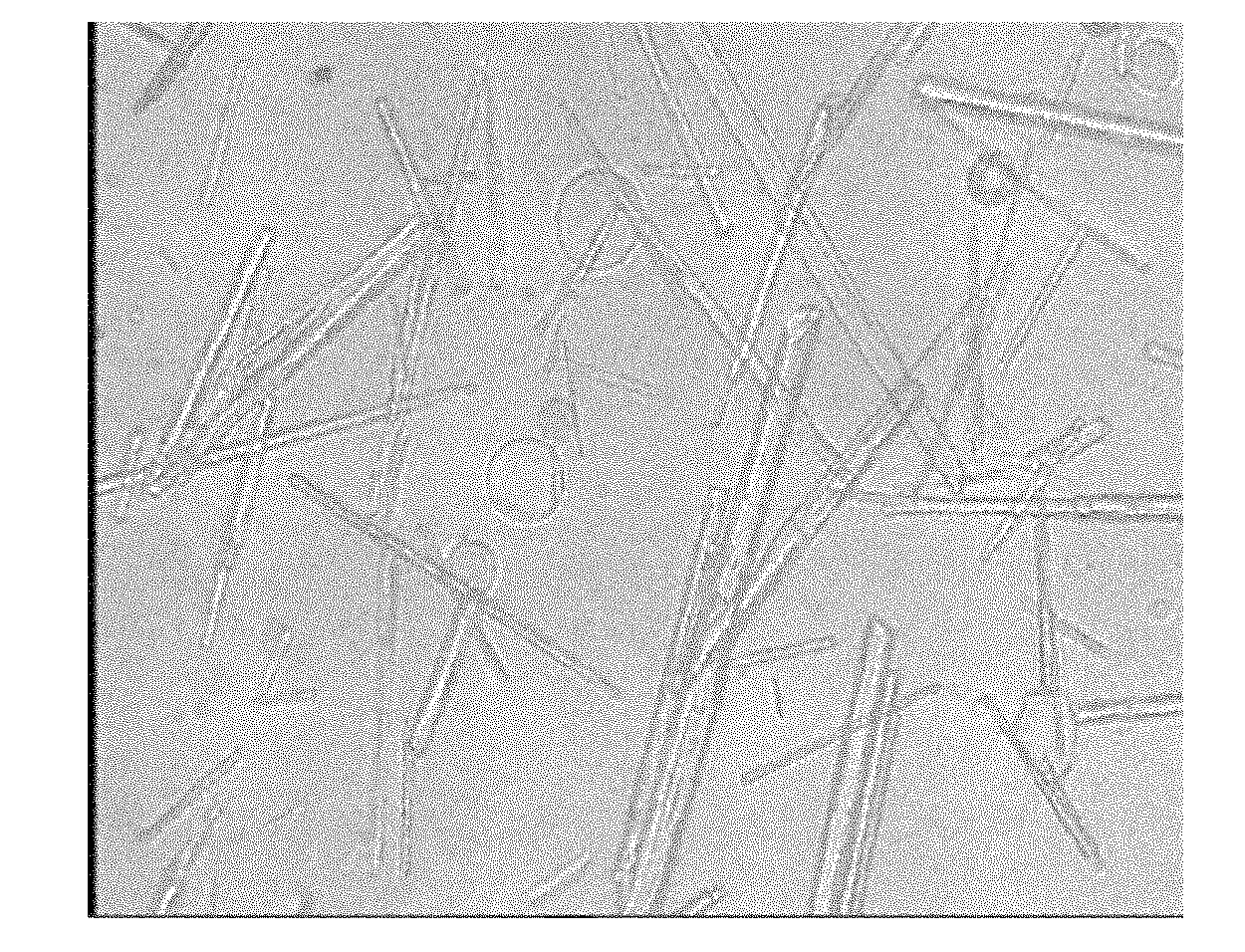
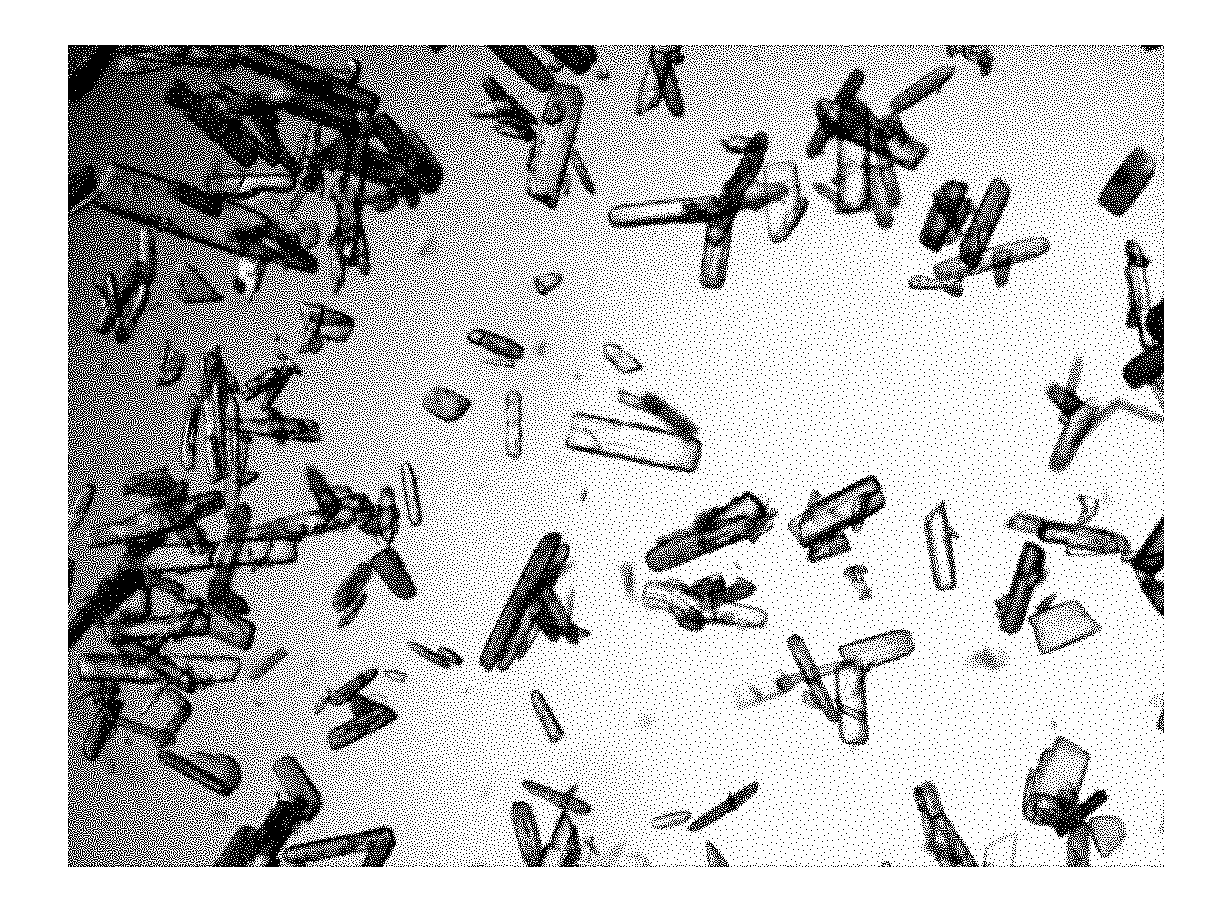
Method for preparing penicillin-G-1-(S)-oxide
A technology of penicillin sulfoxide and penicillin, which is applied in the field of preparation of penicillin sulfoxide, can solve the problems of reduced production efficiency and output, long production cycle, and poor product quality, and achieve the effects of reduced labor intensity, reduced production cycle, and simple operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] Get 500mL penicillin fermented liquid, the penicillin concentration 6g / L in the fermented liquid. Weigh 0.5g of activated carbon into the penicillin fermentation broth, stir for 10 minutes to decolorize, after the decolorization is completed, measure 500mL of butyl acetate and add to the fermentation broth and stir, adjust the pH value of the penicillin fermentation broth to 1.5 with 2mol / L dilute sulfuric acid aqueous solution for extraction. After continuing to stir for 30 minutes, the solution was transferred to a separatory funnel, and the butyl acetate phase was separated from the fermentation broth to obtain a 6 g / L penicillin butyl acetate solution. It was added to the crystallizer and stirred at a constant temperature of -5°C. Measure 7.5mL of peracetic acid solution with a concentration of 8% and cool it down to -5°C, then add it into the crystallizer at a rate of 0.12mL / min to carry out oxidation reaction crystallization. After the dropwise addition of the ox...
Embodiment 2
[0028] Take by weighing 35g penicillin salt, be dissolved in 500mL water, obtain the aqueous solution that penicillin salt concentration is 70g / L. Weigh 1.5 g of activated carbon and add penicillin saline solution, stir and decolorize for 20 minutes. After decolorization is completed, measure 500 mL of ethyl acetate and add penicillin saline solution to stir, and use 1 mol / L dilute sulfuric acid aqueous solution to adjust the pH value of penicillin saline solution to 2.0 for extraction. After continuing to stir for 30 minutes, the solution was transferred to a separatory funnel, and the ethyl acetate phase was separated from the aqueous solution to obtain a 70 g / L penicillin ethyl acetate solution. It was added to the crystallizer and stirred at a constant temperature of 0°C. Measure 46.7mL of peracetic acid solution with a concentration of 30% and cool it down to 0°C, then add it into the crystallizer at a speed of 2.33mL / min to carry out oxidation reaction crystallization. ...
Embodiment 3
[0030] Get 500mL penicillin fermented liquid, the penicillin concentration 30g / L in the fermented liquid. Weigh 2 g of activated carbon and add it to the penicillin fermentation broth, stir and decolorize for 30 minutes. After the decolorization is completed, measure 500 mL of methyl acetate and add it to the fermentation broth and stir. Use 2 mol / L dilute sulfuric acid aqueous solution to adjust the pH of the penicillin fermentation broth to 2.5 for extraction. After continuing to stir for 30 minutes, the solution was transferred to a separatory funnel, and the methyl acetate phase was separated from the fermentation broth to obtain a 30 g / L penicillin methyl acetate solution. It was added to the crystallizer and stirred at a constant temperature of 5°C. Measure 12.5 mL of hydrogen peroxide solution with a concentration of 30% and cool it down to 5°C, then slowly add it into the crystallizer at a rate of 1.25 mL / min to carry out oxidation reaction crystallization. After the ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com