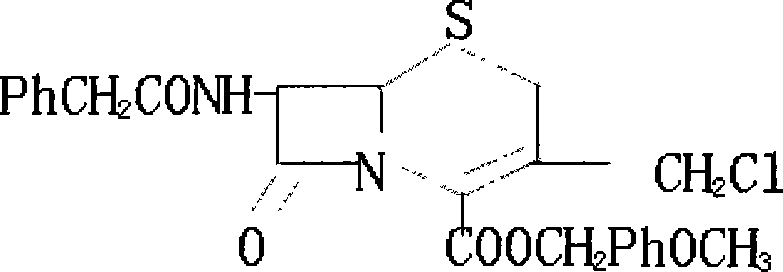
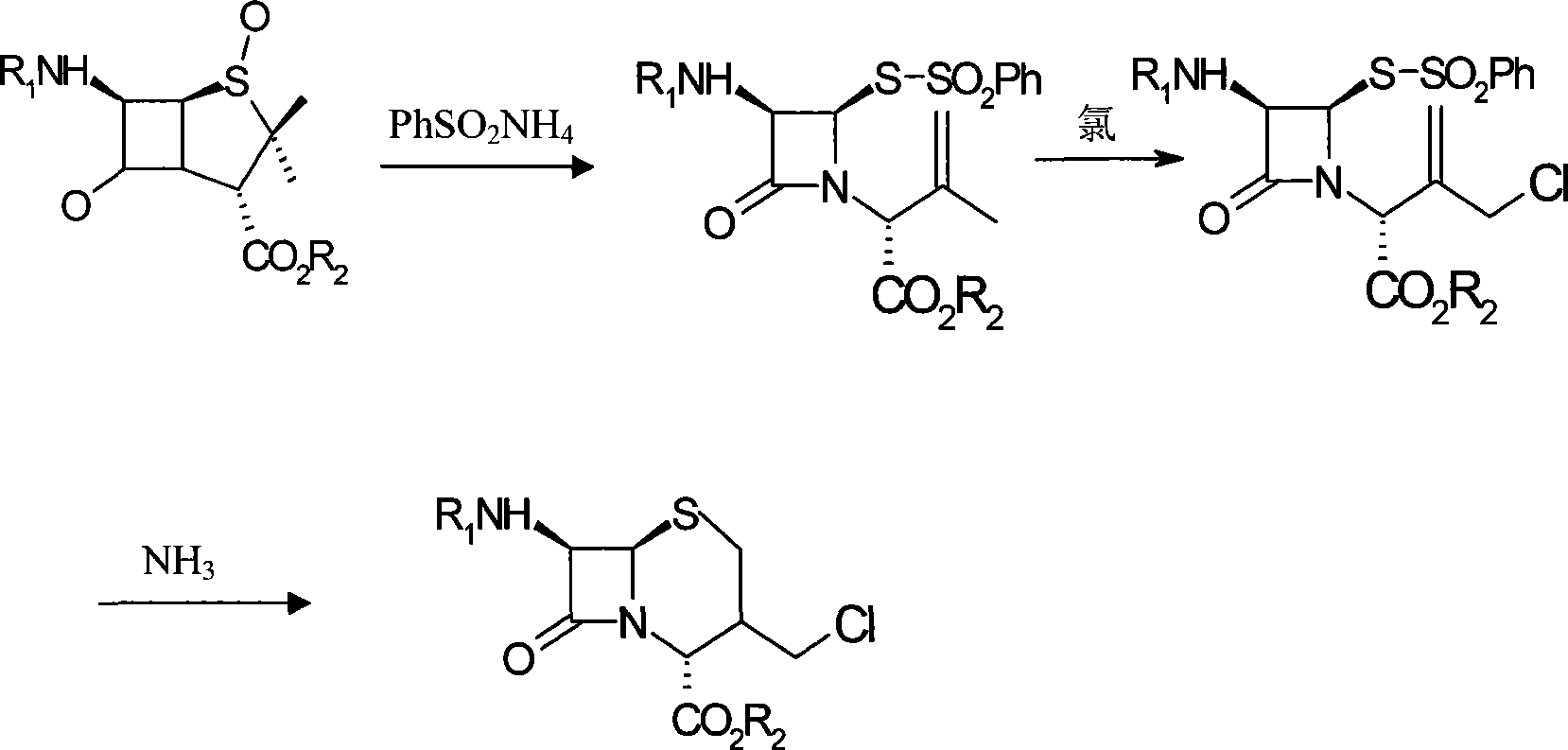
Method for synthesizing 7-phenylacetylamino-3-chloromethyl cephalosporin alkyl acid p-methoxybenzyl ester
A technology of chloromethyl cephalenic acid and p-methoxybenzyl ester, which is applied in the field of cephalosporin antibiotic drug synthesis, can solve the problems of difficult control of reaction conditions, high technical difficulty, influence on yield and the like, and achieves outstanding substantive characteristics , The effect of reducing production cost and easy operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0014] Add 40kg of penicillin sulfoxide ester, 18kg of ammonium benzenesulfinate, and 13kg of 2-mercaptobenzothiazole into the reaction tank, then add 120kg of dichloromethane, stir and heat up to react at 38°C for 4 hours, and under normal pressure at 39°C The solvent was distilled off to generate the azetidinone thiosulfinate intermediate; the temperature of the reaction tank was cooled with tap water for 1 hour, and then 150 kg of dichloromethane was pumped in, and the saturated ice brine was cooled to -15°C with stirring, and then added 30kg of trichloroisocyanic acid was stirred and reacted at -15°C for 1 hour to generate azetidinone thiosulfinate allylic chlorination product; after the reaction solution was evaporated to dryness under reduced pressure at 0.06MPa, di Methylformamide 150kg, start stirring and let saturated ice brine cool down to -15°C, add 15kg of 16% ammonia water, stir and react at -15°C for 2.5 hours, add water 200kg and dichloromethane 150kg to the reac...
Embodiment 2
[0016] Add 40kg of penicillin sulfoxide ester, 18kg of ammonium benzenesulfinate, and 13kg of 2-mercaptobenzothiazole into the reaction tank, then add 120kg of dichloromethane, start stirring and heat up at 40°C for 4.5 hours, and under normal pressure at 42°C The solvent was distilled off to generate the azetidinone thiosulfinate intermediate; the temperature of the reaction tank was cooled with tap water for 1.5 hours, and then 150 kg of dichloromethane was sucked in, and the saturated ice brine was cooled to -20°C with stirring, and added 30kg of trichloroisocyanic acid was stirred and reacted at -20°C for 1.2 hours to generate azetidinone thiosulfinate allylic chlorination product; after the reaction solution was evaporated to dryness under reduced pressure at 0.08MPa, di Methylformamide 150kg, start stirring and let saturated ice brine cool down to -20°C, add 15kg of 18% ammonia water, stir and react at -20°C for 3 hours, add water 200kg and methylene chloride 150kg to the...
Embodiment 3
[0018] Add 40kg of penicillin sulfoxide ester, 18kg of ammonium benzenesulfinate, and 13kg of 2-mercaptophenprothiazole into the reaction tank, then add 120kg of dichloromethane, start stirring and heat up at 39°C for 4.2 hours, and under normal pressure at 40°C The solvent was distilled off to generate the azetidinone thiosulfinate intermediate; the temperature of the reaction tank was cooled by tap water for 1.2 hours, and then 150 kg of dichloromethane was pumped in, and the saturated ice brine was cooled to -18°C with stirring, and added 30kg of trichloroisocyanic acid was stirred and reacted at -18°C for 1.1 hours to generate azetidinone thiosulfinate allylic chlorination product; after the reaction solution was evaporated to dryness under reduced pressure at 0.07MPa, di Methylformamide 150kg, start stirring and let saturated ice brine cool down to -18°C, add 15kg of 17% ammonia water, stir and react at -18°C for 2.8 hours, add water 200kg and dichloromethane 150kg to the ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com