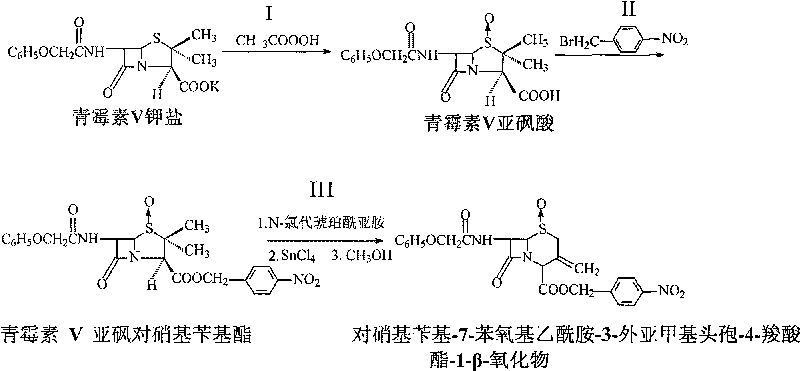
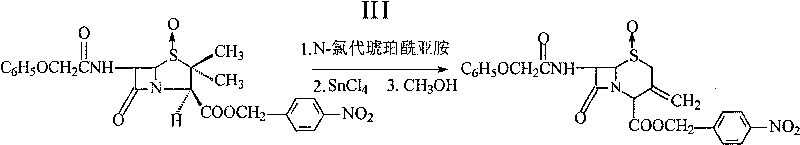
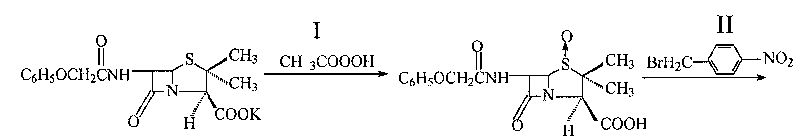Method for preparing p-nitrobenzyl-7-phenoxyacetamido-3-exomethylenecepham-4-carboxylate-1-beta-oxide
A technology of phenoxyacetamide and exomethylene cephalosporin, which is applied in the field of preparing p-nitrobenzyl in the Ming Dynasty, can solve the problems of solvent waste environment, long reaction time, low yield, etc., achieve good effect and improve Reaction conversion rate and yield, effect of high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0016] In a 500ml four-necked bottle, add 50.6ml of peracetic acid with a concentration of 18%, lower the temperature to 0-5°C, dissolve 38.8g of penicillin V potassium salt in 250ml of distilled water, gradually add it dropwise to the four-necked bottle within 2 hours, and keep the temperature 0-5°C, continue to react for 3 hours, raise the temperature to 35°C, add 32ml of 10% sulfuric acid solution, measure the pH value to 1.5, cool down to 0°C, keep it for 0.5 hours, filter, and wash the filter mass with distilled water three times until neutral , vacuum-dried at 50°C to constant weight to obtain 36.2g of penicillin V sulfoxide acid, with a molar yield of 99%, a melting point of 151-152°C, and a purity of 98.7% by liquid chromatography analysis.
[0017] Carboxyl protection reaction: 7.5g of penicillin V sulfoxide acid was added to a 500ml four-necked flask, and 60ml of acetone, 2.88ml of triethylamine, and 4.86g of p-nitrobenzyl bromide were added to the four-necked flask i...
Embodiment 2
[0021] In a 500ml four-necked bottle, add 114ml of peracetic acid with a concentration of 8%, cool down to 0-5°C, dissolve 38.8g of penicillin V potassium salt in 250ml of distilled water, gradually drop into the four-necked bottle within 2 hours, and keep the temperature at 0-5°C, continue to react for 3 hours, raise the temperature to 35°C, add 32ml of sulfuric acid solution with a concentration of 10%, measure the pH value as 1.5, cool down to 0°C, keep for 0.5 hours, filter, wash the filter mass with distilled water three times until neutral, Vacuum drying at 50°C to constant weight yielded 34.6g of penicillin V sulfoxide acid with a molar yield of 94.5%, a melting point of 151-152°C, and a purity of 98.5% by liquid chromatography analysis.
[0022] Carboxyl protection reaction: 7.5g of penicillin V sulfoxide acid was added to a 500ml four-necked flask, and 60ml of acetone, 3.42ml of triethylamine, and 5.31g of p-nitrobenzyl bromide were successively added to the four-necke...
Embodiment 3
[0026] In a 500ml four-necked bottle, add 65.1ml of peracetic acid with a concentration of 14%, lower the temperature to 0-5°C, dissolve 38.8g of penicillin V potassium salt in 250ml of distilled water, gradually add it dropwise to the four-necked bottle within 2 hours, and keep the temperature 0-5°C, continue to react for 3 hours, raise the temperature to 35°C, add 35ml of 10% sulfuric acid solution, measure the pH value to 1.5, cool down to 0°C, keep it for 0.5 hours, filter, and wash the filter mass with distilled water three times until neutral , vacuum-dried at 50°C to constant weight to obtain 35.4g of penicillin V sulfoxide acid, with a molar yield of 96.7%, a melting point of 151-152°C, and a purity of 98.7% by liquid chromatography analysis.
[0027] Carboxyl protection reaction: 7.5g of penicillin V sulfoxide acid was added to a 500ml four-necked flask, and 60ml of acetone, 3.1ml of triethylamine, and 4.6g of p-nitrobenzyl bromide were successively added to the four-n...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
| purity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com