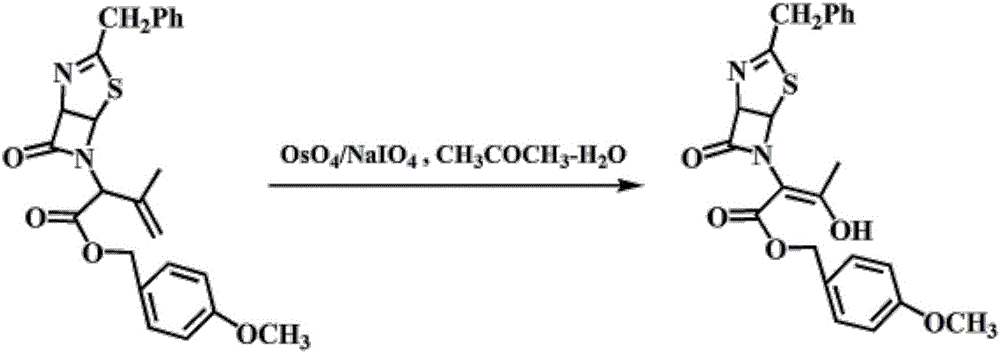Method for preparing thiazoline enol ester
A technology of thiazoline enol ester and thiazoline, which is applied in the field of preparation of thiazoline enol ester, can solve the problems of harsh conditions, high energy consumption, and low yield, and achieve low cost, high yield, and simple operation Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] A kind of preparation method of thiazoline enol ester, specifically comprises the steps:
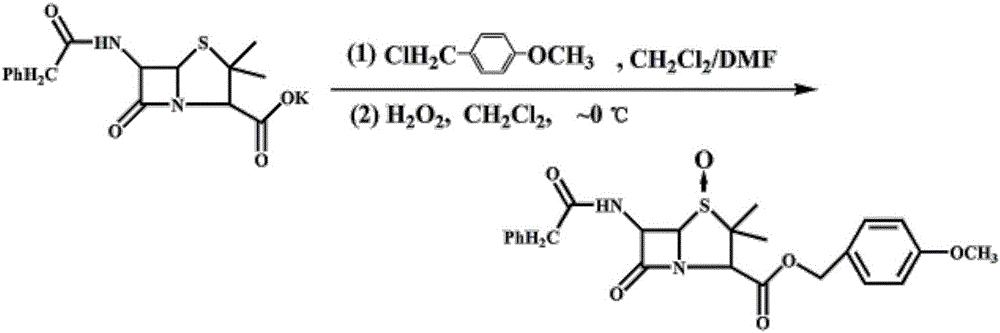
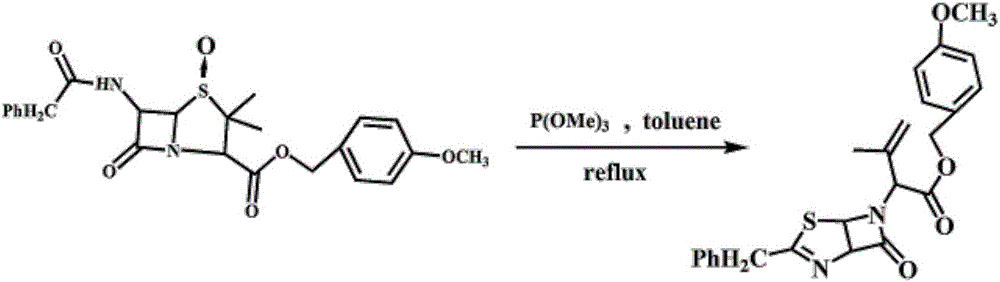
[0026] ① Preparation of penicillin sulfoxide ester: Add 18.6g of penicillin G potassium salt, 28mL of N,N-dimethylformamide (DMF), 100mL of dichloromethane, 9.4g of p-methoxybenzyl chloride, four Butylammonium bromide 0.56g, heated to reflux for 6 hours, monitored by TLC, cooled to 0°C, under nitrogen protection, then added 200mL of dichloromethane, then added dropwise 7.6g of 35% hydrogen peroxide, after the dropwise addition, stirred for 30 minutes , then add 1.6 g of maleic anhydride, TLC monitoring, the reaction is complete, control the temperature below 10 ° C, dropwise add 7% sodium sulfite solution, use starch-potassium iodide test paper to test that the feed liquid does not change color, and it is the end point of the dropwise addition. After the dropwise addition is completed, stir for 15 Minutes, standing for layering, the organic phase was washed with 50mL of 5% sodium ...
Embodiment 2
[0030] A kind of preparation method of thiazoline enol ester, specifically comprises the steps: 1. the preparation of penicillin sulfoxide ester: in reactor, add penicillin G potassium salt 18.6g, N,N-dimethylformamide (DMF) 28mL, Dichloromethane 100mL, p-methoxybenzyl chloride 9.4g, tetrabutylammonium bromide 0.56g, heating to reflux for 6 hours, TLC monitoring, cooling to 0°C, under nitrogen protection, then adding 200mL of dichloromethane, then drop Add 7.6g of 35% hydrogen peroxide, after the dropwise addition, stir for 30 minutes, then add 1.6g of maleic anhydride, monitor by TLC, after the reaction is complete, control the temperature below 10°C, add 7% sodium sulfite solution dropwise, and test with starch-potassium iodide test paper The feed liquid does not change color and is the end point of the dropwise addition. After the dropwise addition is completed, stir for 15 minutes, let stand and separate layers, wash the organic phase with 50mL of 5% sodium bicarbonate solu...
Embodiment 3
[0034] A kind of preparation method of thiazoline enol ester, specifically comprises the following steps: 1. the preparation of penicillin sulfoxide ester: in reactor, add penicillin G potassium salt 18.6g, N,N-dimethylformamide (DMF) 50mL, Dichloromethane 100mL, p-methoxybenzyl chloride 7.9g, tetrabutylammonium bromide 0.37g, heating to reflux for 6 hours, TLC monitoring, cooling to 0 ° C, under nitrogen protection, then adding 200mL of dichloromethane, and then drop Add 6.6g of 35% hydrogen peroxide, after the dropwise addition, stir for 30 minutes, then add 1.6g of maleic anhydride, monitor by TLC, after the reaction is complete, control the temperature below 10°C, add 7% sodium sulfite solution dropwise, and test with starch-potassium iodide test paper The feed liquid does not change color and is the end point of the dropwise addition. After the dropwise addition is completed, stir for 15 minutes, let stand and separate layers, wash the organic phase with 50mL of 5% sodium ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com