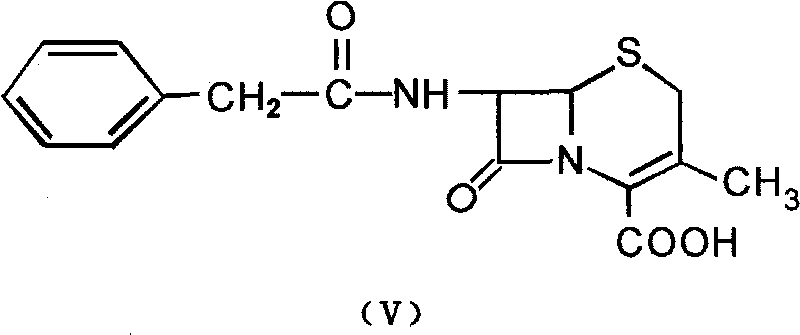
Method for preparing cephalosporin intermediate 7-ADCA
A 7-ADCA and cephalosporin technology, applied in the field of compound preparation, can solve the problems of insufficient yield, poor quality, and low yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
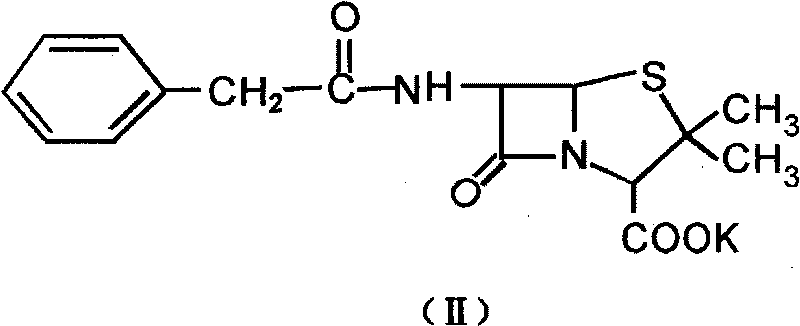
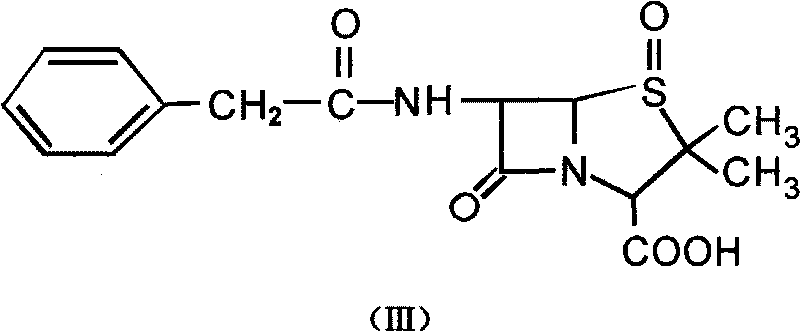
[0023] The first step: the preparation of penicillin G sulfoxide (III)
[0024] 1. Preparation of Oxidant Peracetic Acid
[0025] Add 100g (1.67mol) of glacial acetic acid and 4.7g (0.047mol) of 98% concentrated sulfuric acid into the reactor, and slowly add 92.5g (0.82mol) of 30% hydrogen peroxide dropwise at 30 to 35°C under stirring. Stir at room temperature for 5h and store at low temperature.
[0026] 2. Oxidation process
[0027] Add 37.25g (0.1mol) of penicillin G potassium into the reactor, then add 25mL of water to dissolve it, cool it down to 0°C in an ice bath, measure 50g (0.1mol, C=15%) of peracetic acid, and add it to the reaction In the container (with a mechanical stirring device), use potassium acetate solid to adjust the PH process and carry out HPLC analysis by sampling every half an hour to track the reaction of raw materials. Precipitate, stand in an ice-water bath for 0.5h, filter, wash the filter cake with ice water, until Ba(NO 3 ) 2 Solution inspe...
Embodiment 2
[0043] The first step: the preparation of penicillin G sulfoxide (III)
[0044] 1. Preparation of Oxidant Peracetic Acid
[0045] Add 100g (1.67mol) of glacial acetic acid and 4.7g (0.047mol) of 98% concentrated sulfuric acid into the reactor, and slowly add 92.5g (0.82mol) of 30% hydrogen peroxide dropwise at 30 to 35°C under stirring. Stir at room temperature for 5h and store at low temperature.
[0046] 2. Oxidation process
[0047] Add 37.25g (0.1mol) of penicillin G potassium into the reactor, then add 25mL of water to dissolve it, cool it down to 0°C in an ice bath, measure 50g (0.1mol, C=15%) of peracetic acid, and add it to the reaction In the container (with a mechanical stirring device), use potassium acetate solid to adjust the PH process and carry out HPLC analysis by sampling every half an hour to track the reaction of raw materials. Precipitate, stand in an ice-water bath for 0.5h, filter, wash the filter cake with ice water, until Ba(NO 3 ) 2 Solution inspe...
Embodiment 3
[0063] The first step: the preparation of penicillin G sulfoxide (III)
[0064] 1. Preparation of Oxidant Peracetic Acid
[0065] Add 100g (1.67mol) of glacial acetic acid and 4.7g (0.047mol) of 98% concentrated sulfuric acid into the reactor, and slowly add 92.5g (0.82mol) of 30% hydrogen peroxide dropwise at 30 to 35°C under stirring. Stir at room temperature for 5h and store at low temperature.
[0066] 2. Oxidation process
[0067] Add 37.25g (0.1mol) of penicillin G potassium into the reactor, then add 25mL of water to dissolve it, cool it down to 0°C in an ice bath, measure 50g (0.1mol, C=15%) of peracetic acid, and add it to the reaction In the container (with a mechanical stirring device), use potassium acetate solid to adjust the PH process and carry out HPLC analysis by sampling every half an hour to track the reaction of raw materials. Precipitate, stand in an ice-water bath for 0.5h, filter, wash the filter cake with ice water, until Ba(NO 3 ) 2 Solution inspe...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com