Penicillin G sulfoxide diphenyl methyl ester synthesis method
A technology of diphenylmethyl sulfoxide and synthesis method, applied in the direction of organic chemistry, etc., can solve the problems of peracetic acid flammability, low safety factor, unfavorable operation, etc., achieve effective non-chlorine oxidation ability, high safety factor, easy The effect of the operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
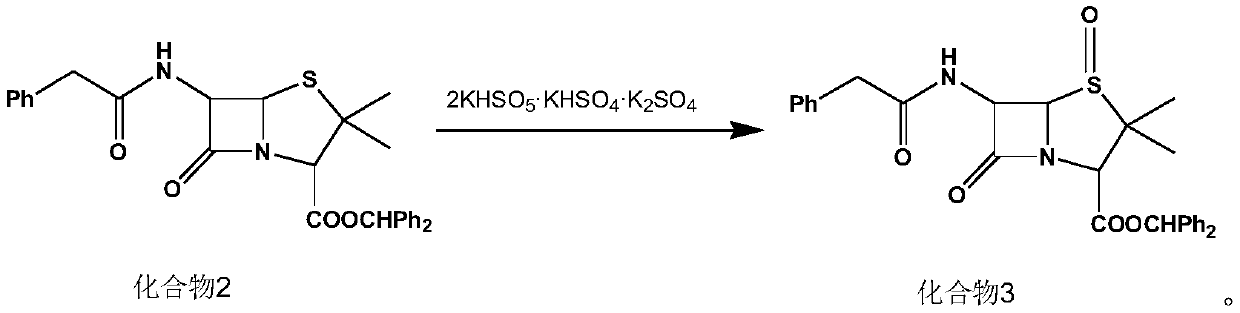
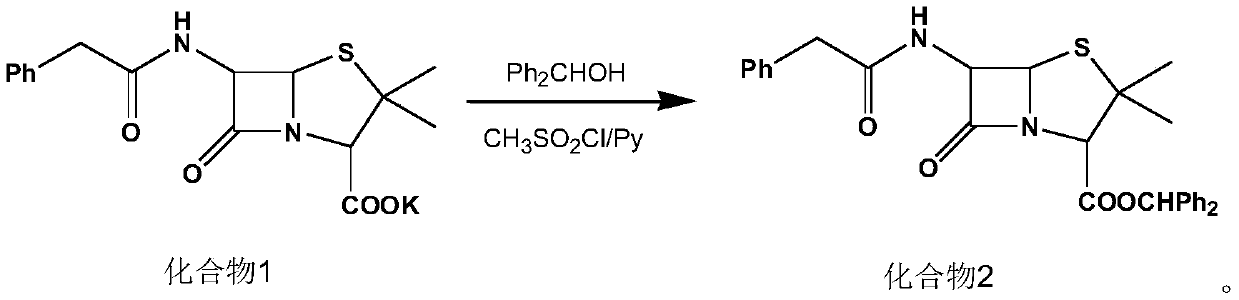
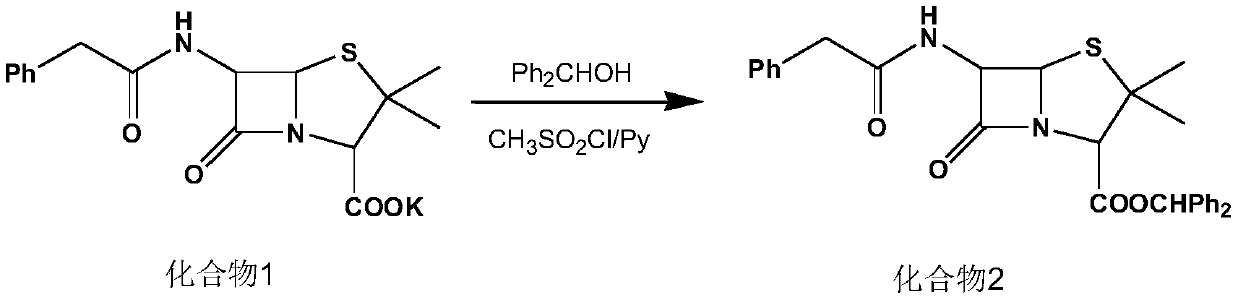
[0028] (1) Synthesis of compound 2:
[0029] Add 120ml of dichloromethane, diphenylmethanol (23.2g, 0.126mol) and compound 1 (40g, 0.107mol) to a 500ml four-neck flask, cool down to 15°C, add pyridine (16g, 0.202mol), and control the temperature for 10-15°C. Add methanesulfonyl chloride (16 g, 0.140 mol) dropwise at ℃ for 60 minutes, and keep warm for 4 hours after dropping. At the end of the heat preservation, 135 g of 5% dilute sulfuric acid was added for washing, the organic phase was separated, the aqueous phase was extracted with 30 ml of dichloromethane, and the organic phases were combined to obtain a dichloromethane solution of compound 2, which was then transferred to a 1000 ml four-necked bottle for use.
[0030] (2) Synthesis of compound 3:
[0031] Adjust the temperature of the dichloromethane solution of compound 2 prepared in the previous step to 22°C. Preparation of potassium persulfate complex salt solution: weigh potassium persulfate complex salt (45 g, 0.07...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com