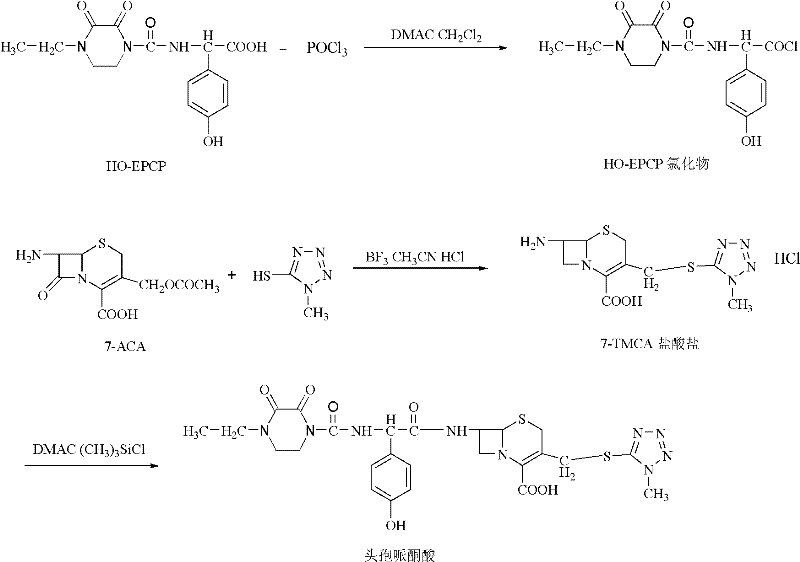
Synthesis method of cefoperazone acid
A technology of cefoperazone acid and synthesis method, which is applied in the field of medicine, can solve the problems of harsh reaction conditions, long reaction time, and high cost of raw materials, and achieve the effects of reducing side reactions and increasing reaction yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] 1. Preparation of 7-TMCA hydrochloride
[0026] Under nitrogen, dissolve 15.4g of 1-methyl-5-mercapto-1,2,3,4-tetrazolium into 157.0mL of boron trifluoride acetonitrile, keep the reaction for 1h, and add 36.0g of 7-ACA three times , stirred at 25-30°C for 2h, then added 80mL of concentrated hydrochloric acid dropwise within 0.5h, added 80mL of water, kept at 15°C, stirred for 2h, stirred at 5°C for 1h, suction filtered, and the filter cake was washed with acetonitrile-acetone (1:1 V / V) 40 mL was washed three times, and vacuum-dried at 48-50° C. to obtain 46.10 g of white crystals.
[0027] 25.0g of 7-TMCA hydrochloride and 80mL of N,N-dimethylacetamide were stirred and dissolved, and 6mL of trimethylchlorosilane was added dropwise under cooling in an ice bath, and stirred at 15-20°C for 1h to obtain a 7-TMCA solution , which is the solution ①.
[0028] 2. Preparation of HO-EPCP chloride
[0029] Add 140.0mL N,N-dimethylacetamide, 145.2mL CH 2 Cl 2 , 26.9gHO-EPCP, ...
Embodiment 2
[0033] 1. Synthesis of 7-TMCA hydrochloride
[0034] In a 500mL round bottom flask, add 165mL boron trifluoride acetonitrile, 37g 7-ACA and 15.6g 1-methyl-5-mercapto-1,2,3,4-tetrazolium in sequence, stir rapidly, and the reaction temperature is 25 ~30°C, reaction time 2.5h, then add 85mL of concentrated hydrochloric acid dropwise within 0.5h, add 75mL of water, keep warm at 15°C, stir for 1.5h, filter with suction, wash the crystal with 40mL of acetonitrile-acetone (1:1 V / V) for 3 The second time, 45.8 g of white crystals were obtained by vacuum drying at 48-50°C.
[0035] 25.0g of 7-TMCA hydrochloride and 80mL of N,N-dimethylacetamide were stirred and dissolved, and 6mL of trimethylchlorosilane was added dropwise under cooling in an ice bath, and stirred at 15-20°C for 1h to obtain a 7-TMCA solution , which is the solution ①.
[0036] 2. Preparation of HO-EPCP chloride
[0037] Add 138.0mL DMAC, 146.0mL CH 2 Cl 2, 27.2g HO-EPCP, stirred until dissolved. Cool down to -20...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com