Method for synthesizing cephalosporin intermediate
A synthesis method and intermediate technology are applied in the field of synthesis of cephalosporin intermediates, can solve the problem that the synthesis method of cephalosporin intermediates is not suitable for industrial production, etc., and achieve the effects of good solubility, convenient operation and mild reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] In the present embodiment, a kind of synthetic method of cephalosporin intermediate adopts the following steps:
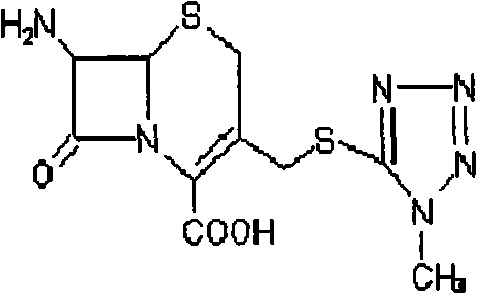
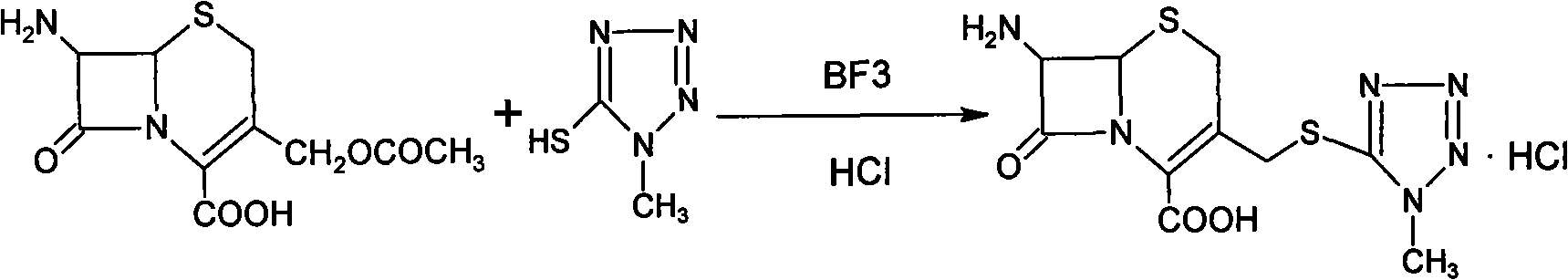
[0030] 1. Add 100ml of acetonitrile to a 500ml three-necked flask, add 10g of 1-methyl-5-mercaptotetrazolium into the acetonitrile, and add 20g of 7-aminocephalosporanic acid while stirring; the reaction solution is heated to 50 ℃, add 110ml boron trifluoride acetonitrile solution with 20% boron trifluoride content, keep stirring at this temperature for 2h.
[0031] 2. After the reaction is over, cool the reaction solution to room temperature, add 3 g of activated carbon, and stir for 15 minutes. Filter, wash the charcoal with 150 ml of acetone aqueous solution (V acetone / V water=1 / 1) containing 0.5% hydrochloric acid, and the washing liquid is combined with the filtrate.
[0032] 3. Add the combined filtrate to 100ml acetone aqueous solution (V acetone / V water=1 / 1), stir, slowly add 8% sodium bicarbonate solution dropwise until the pH value of the solution...
Embodiment 2
[0036] In the present embodiment, a kind of synthetic method of cephalosporin intermediate adopts the following steps:
[0037] 1. Add 100ml of acetonitrile to a 500ml three-necked flask, add 10g of 1-methyl-5-mercaptotetrazolium into the acetonitrile, and add 20g of 7-aminocephalosporanic acid while stirring; the reaction solution is heated to 25 ℃, add 35ml of boron trifluoride ether solution with boron trifluoride content of 47%, and keep stirring at this temperature for 1 h.
[0038] 2. After the reaction is finished, cool the reaction solution to room temperature, add 3 g of activated carbon to the solution, and stir for 30 minutes. Filter, wash the charcoal with 25 ml of acetone aqueous solution containing 5% hydrochloric acid (V acetone / V water=1 / 4), and the washing liquid is combined with the filtrate.
[0039] Three, the combined filtrate is added in 200ml acetone aqueous solution (V acetone / V water=1 / 4), stir, slowly add dropwise 10% sodium carbonate solution to sol...
Embodiment 3
[0043] In the present embodiment, a kind of synthetic method of cephalosporin intermediate adopts the following steps:
[0044] 1. Add 250ml of acetonitrile into a 1000ml three-necked flask, add 15g of 1-methyl-5-mercaptotetrazolium into the acetonitrile, add 29g of 7-aminocephalosporanic acid while stirring, and heat the reaction solution to 35°C , add 40ml of boron trifluoride tetrahydrofuran solution with a boron trifluoride content of 47%, keep the temperature and stir for 2h;
[0045]2. After the reaction, cool the reaction solution to room temperature, add 5 g of activated carbon, stir for 20 min, and filter; wash the carbon with 20 ml of acetone aqueous solution (V acetone / V water=4 / 1) containing 10% hydrochloric acid, and the washing liquid and the filtrate are combined .
[0046] 3. Add the combined filtrate to 120ml acetone aqueous solution (V acetone / V water=4 / 1), stir, slowly add 15% sodium hydroxide solution dropwise until the pH value of the solution is 2.0, sto...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com