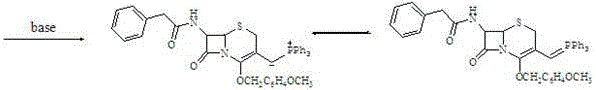
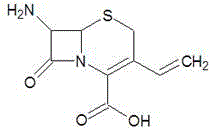
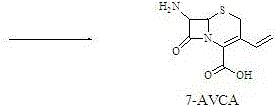
7-amino-3-vinyl cephalosporanic acid preparation method
A technology based on cephalosporanic acid and vinyl, which is applied in the field of preparation of 7-amino-3-vinyl cephalosporanic acid, can solve the problems of poor product purity, many side reactions, and difficulty in solvent recovery, and achieve good quality and high-quality products. The effect of high yield and less waste
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035]Add 400ml of chloroform and 400ml of water to the reaction bottle, and add 60g (123.2mmol) of 7-phenylacetamido-3-chloromethyl cephalosporanic acid p-methoxybenzyl ester (GCLE), 18.5g (123.2mmol) of Sodium iodide and 34g (129.6mmol) triphenylphosphine were maintained at 30-35°C for reaction, and the reaction progress was monitored by HPLC. After 2 hours, the reaction was basically completed. Separate layers, lower the temperature of the lower organic phase to 0-5°C, add 400ml of 1.6% aqueous sodium hydroxide solution (w / w), and keep stirring at 0-5°C for 0.5h. The layers were separated, the upper aqueous phase was retained and used mechanically as the aqueous phase containing sodium iodide, and the lower organic phase was washed with 400ml of water. Add 75ml of 37% formaldehyde aqueous solution (w / w) and 4g of tetrabutylammonium bromide to the organic phase, stir thoroughly at 0-10°C, monitor the reaction progress with HPLC, and the reaction is basically completed after ...
Embodiment 2
[0037] Transfer the aqueous phase containing sodium iodide to be applied mechanically into the reaction flask, adjust the pH to 6-7 with 10% hydrochloric acid, add 400ml of chloroform, 60g (123.2mmol) of 7-phenylacetamido-3-chloromethylcephalosporanic acid p-Methoxybenzyl ester (GCLE), 34g (129.6mmol) triphenylphosphine, maintain the reaction at 30-35°C, monitor the reaction progress with HPLC, and the reaction is basically completed after 2h. Separate layers, lower the temperature of the lower organic phase to 0-5°C, add 400ml of 1.6% aqueous sodium hydroxide solution (w / w), and keep stirring at 0-5°C for 0.5h. The layers were separated, the upper aqueous phase was retained and used mechanically as the aqueous phase containing sodium iodide, and the lower organic phase was washed with 400ml of water. Add 10ml of formaldehyde-containing aqueous phase and 37% formaldehyde aqueous solution (w / w) to the organic phase, stir thoroughly at 0-10°C, monitor the reaction progress with ...
Embodiment 3
[0039] Transfer the sodium iodide-containing aqueous phase to be applied mechanically into the reaction flask, adjust the pH to 6-7 with 10% hydrochloric acid, add 400ml of dichloromethane, 60g (123.2mmol) of 7-phenylacetamido-3-chloromethyl cephalosporin P-methoxybenzyl alkanoate (GCLE) and 34g (129.6mmol) triphenylphosphine were maintained at 30-35°C for the reaction, and the reaction progress was monitored by HPLC. After 2 hours, the reaction was basically completed. Separate layers, lower the temperature of the lower organic phase to 0-5°C, add 400ml of 1.6% aqueous sodium hydroxide solution (w / w), and keep stirring at 0-5°C for 0.5h. The layers were separated, the upper aqueous phase was retained and used mechanically as the aqueous phase containing sodium iodide, and the lower organic phase was washed with 400ml of water. Add 140ml of 20% formaldehyde aqueous solution (w / w) and 4g of tetrabutylammonium bromide to the organic phase, stir thoroughly at 0-10°C, monitor the ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com