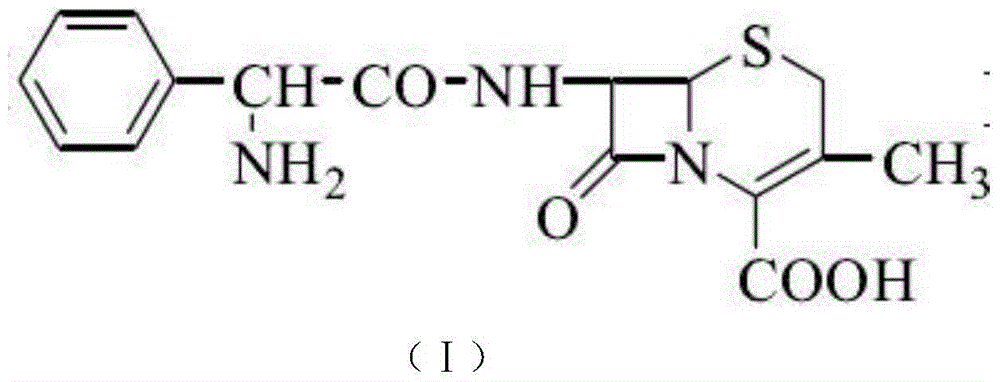
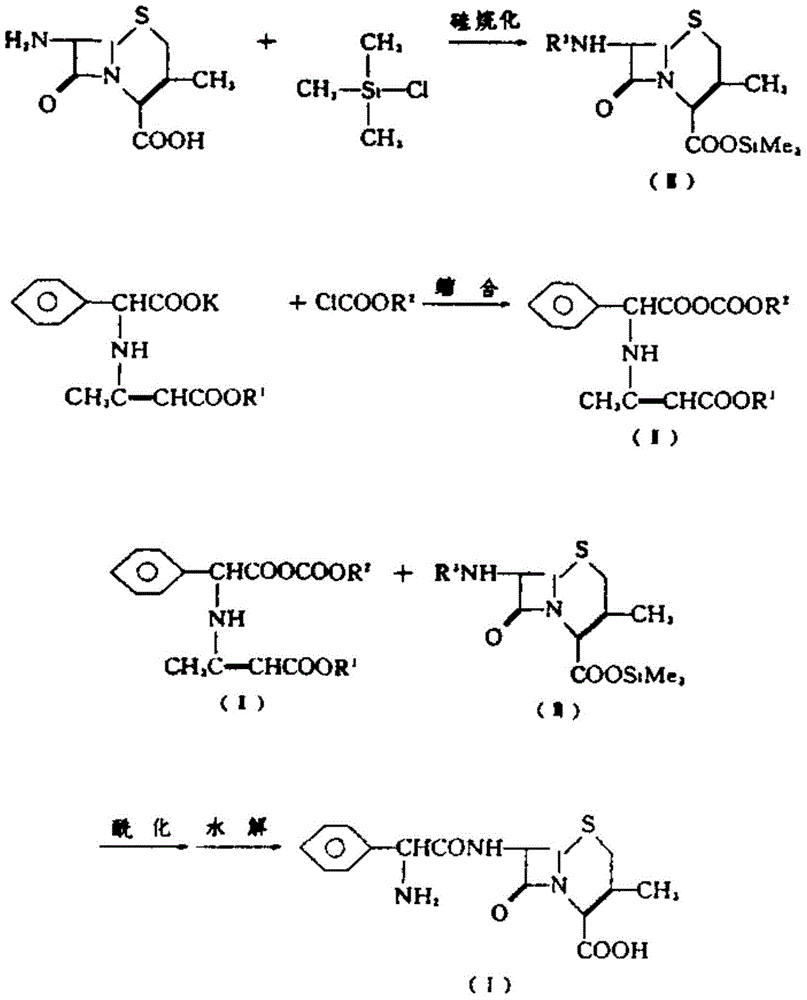
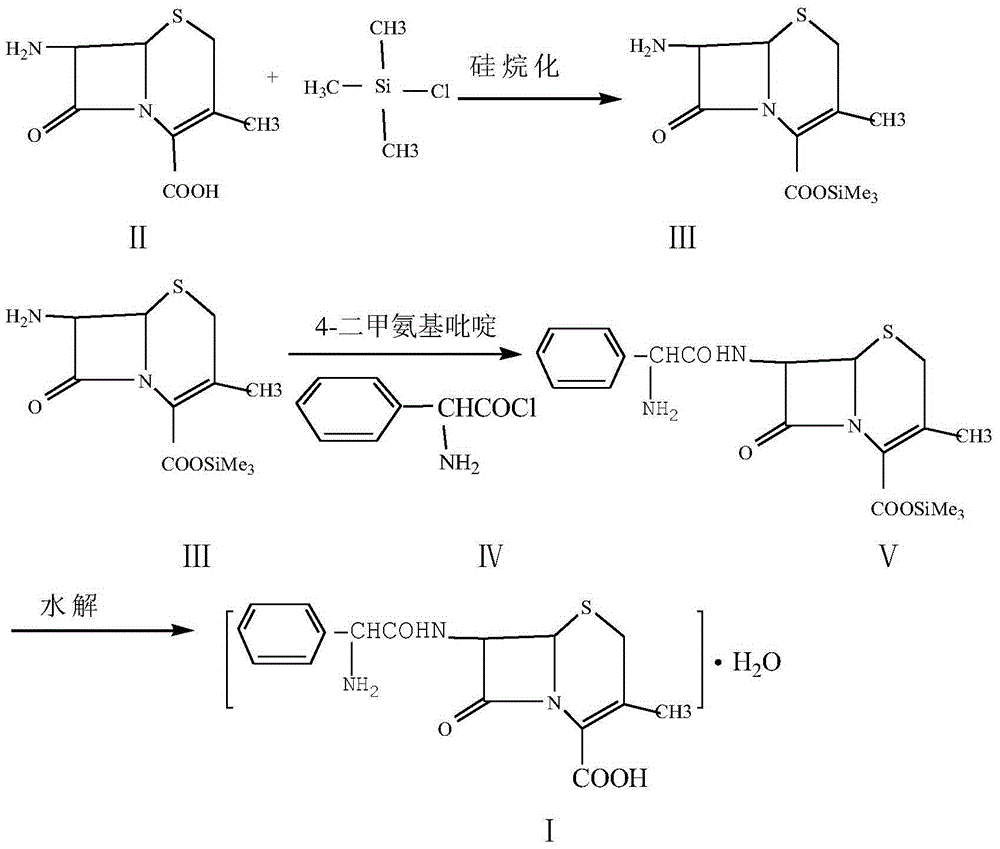
Improvement method of cefalexin synthesis process
A technology of cephalexin and synthesis process, applied in the direction of organic chemistry and the like, can solve the problems of many impurities in the product, complicated reaction process, difficult purification of the product, etc., and achieves the effects of not easy side reactions, high yield, and removal of impurities.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0039] a. Carboxyl protection of 7-ADCA
[0040] Weigh 69g (0.322mol) of 7-ADCA and 1200ml of dichloromethane into a 2L three-necked reaction flask, add 91.5ml of triethylamine under stirring, add 90ml (0.7mol) of trimethylchlorosilane dropwise at 20°C, and react at 20°C After 0.5h, the silyl compound (II) of 7-ADCA was obtained, and the temperature was lowered to -5°C for later use.
[0041] b. Preparation of cephalexin
[0042] Add 60g (0.354mol) of α-aminophenylacetyl chloride, 1500ml of dichloromethane, and 8.5g (0.07mol) of 4-dimethylaminopyridine (DMAP) into the reaction flask, stir and cool down to -5°C, and slowly add step 7-ADCA silyl compound (II) dichloromethane solution, heat and stir at -5°C for 2.5 hours, then add 6ml of triethylamine, continue stirring and reacting for 1.5 hours, take samples for measurement, the residual amount of 7-ADCA in the reaction solution should not exceed 2% is the end point of the reaction.
[0043] Then add 600ml of deionized water...
Embodiment 2
[0048] a. Carboxyl protection of 7-ADCA
[0049] Weigh 69g (0.322mol) of 7-ADCA and 1500ml of dichloromethane into a 2L three-necked reaction flask, add DMF110ml under stirring, add 95ml of 2-(trimethylsilyl)ethoxymethyl chloride dropwise at 20°C, and add at 20°C After reacting for 0.5h, the silyl compound (II) of 7-ADCA was obtained, and the temperature was lowered to -5°C for later use.
[0050] b. Preparation of cephalexin
[0051] Add 60g (0.354mol) of α-aminophenylacetyl chloride, 1500ml of dichloromethane, and 9g of 4-dimethylaminopyridine (DMAP) into the reaction flask, stir and cool down to -10°C, and slowly add the silane of step 7-ADCA dropwise Compound (II) dichloromethane solution, heat and stir at -5°C for 2.5 hours, then add 8.5ml of ethylenediamine, continue to stir and react for 1.5 hours, take samples for measurement, and take the 7-ADCA residue in the reaction solution as not more than 2%. end.
[0052] Then add 650ml of deionized water and concentrated hy...
Embodiment 3
[0056] a. Carboxyl protection of 7-ADCA
[0057] Weigh 69g (0.322mol) of 7-ADCA and 1500ml of chloroform into a 2L three-necked reaction flask, add 110ml of trimethylamine under stirring, add 100ml of tert-butyldiphenylchlorosilane (TbDPSCl) dropwise at 20°C, and react at 20°C After 0.5h, the silyl compound (II) of 7-ADCA was obtained, and the temperature was lowered to -5°C for later use.
[0058] b. Preparation of cephalexin
[0059] Add 72.5g (0.35mol) of α-aminophenylacetyl chloride hydrochloride, 1500ml of chloroform, and 10g of 4-dimethylaminopyridine (DMAP) into the reaction flask, stir and cool down to -10°C, and slowly add step 7 -ADCA silyl compound (II) trichloromethane solution, heat and stir at -5°C for 2.5 hours, then add 8.5ml triethylamine, continue stirring and reacting for 1.5 hours, take samples for measurement, the residual amount of 7-ADCA in the reaction solution should not exceed 2% is the end point of the reaction.
[0060] Then add 720ml of deionize...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com