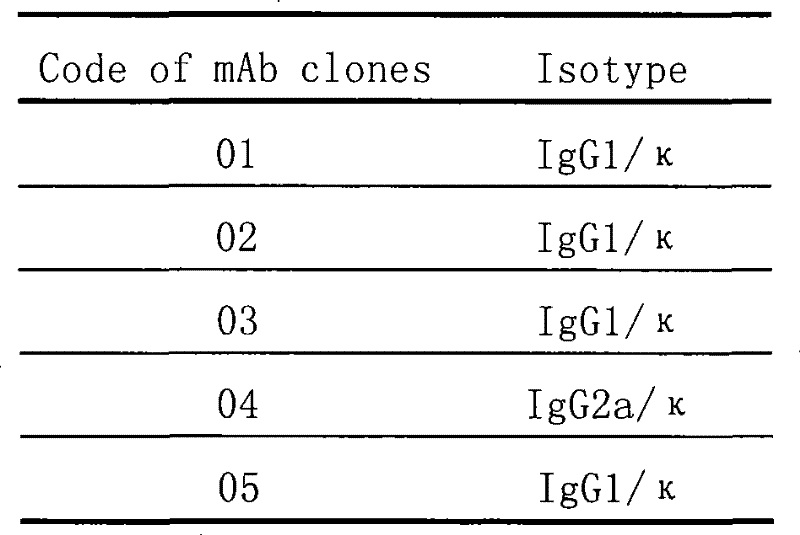
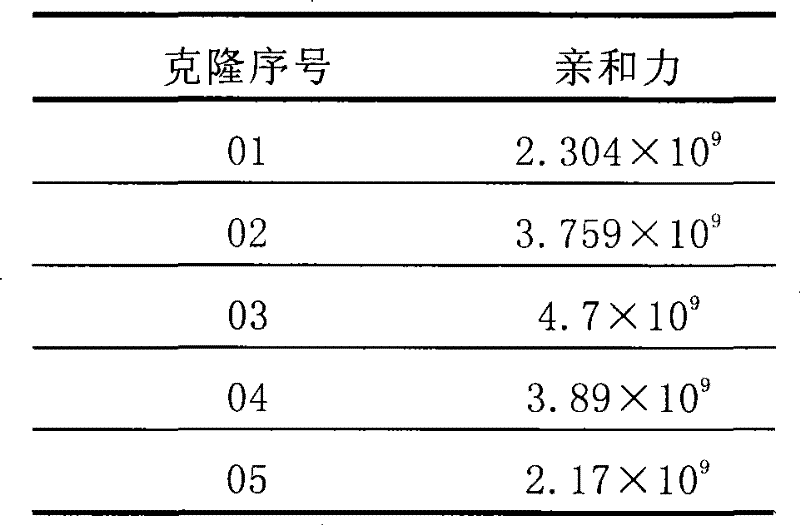
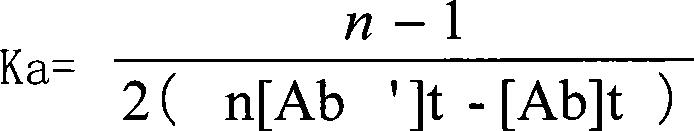Mouse monoclonal antibody cell strain for resisting amoxicillin and ampicillin
A technology of ampicillin mice and monoclonal antibodies, which is applied in chemical instruments and methods, biochemical equipment and methods, instruments, etc., and can solve the problems of high cost, poor timeliness, and high technical requirements
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0014] Example 1: Antigen cross-linking
[0015] 1. Immunogen synthesis:
[0016] Carbodiimide hydrochloride (EDCoHCl) two-step method: Weigh 10-20mg of amoxicillin, 50-150mg of EDCoHC, 5-20mg of NHS (N-hydroxysuccinimide) into a one-mouth bottle, add Fully dissolve in 1-4mL DMF (dimethylformamide), put into the rotor of a magnetic stirrer, seal and avoid light, and react at room temperature for 10-20hrs to obtain amoxicillin reaction solution, which is called A solution. Then weigh 10-50 mg of KLH (keyhole limpet hemocyanin) and fully dissolve it in 1-8 mL of PBS (phosphate buffered saline), called solution B. Then add solution A to solution B dropwise, seal it away from light, and stir overnight at 2-8°C. After the above reaction is completed, the reaction solution is transferred to a dialysis bag pretreated with PBS for 10-32 hrs, and dialyzed with PBS for 12-72 hrs in a refrigerator at 2-8°C with stirring, and the medium is changed several times in between. The dialyzed...
Embodiment 2
[0019] Example 2: Preparation of Monoclonal Antibody
[0020] 1. Immunization of mice and titer determination
[0021] Use amoxicillin-KLH cross-linked product as immunization antigen, immunize Balb / c pure line mice, take antigen at 30-150μg / mouse, mix with Freund's complete adjuvant in equal amount, after fully emulsification, mouse neck Multiple subcutaneous injections on the back. The second immunization was carried out 3 weeks after the first immunization, with the same dose as the first one, emulsified with Freund's incomplete adjuvant, and injected subcutaneously at multiple points on the back of the mouse. Three weeks after the second immunization, the same dose of antigen was dissolved in saline and injected intraperitoneally. 5-15 days after the end of immunization, blood was collected from the tail of the mice, the serum was separated, and the titer of antiserum was determined by indirect ELISA.
[0022] Method for determining antibody titer by indirect ELISA: dil...
Embodiment 3
[0043] Example 3: Production and affinity purification of monoclonal antibodies
[0044] 1. Production of monoclonal antibody
[0045] To ensure the purity of the antibody, the hybridoma cells were cultured in a serum-free medium (Hyclone product, catalog number SH30800). Cells were cultured routinely (cell density was 5-10×10 5 When subcultured, the inoculation density was 1-2×10 5 ), after expanding to 1 L, the culture medium was not changed until about 10 days later, all the hybridoma cells died, and the supernatant was collected by centrifugation, which contained highly specific antibodies.
[0046] 2. Protein G affinity purification
[0047] The Protein G chromatography column (Bio-Rad, Bio-Rad) used for antibody purification was pre-equilibrated with 3-5 column bed volume equilibration buffer (10 mM PBS, pH7.4). The collected supernatant was filtered with a disposable 0.22 μm filter and loaded onto the Protein G chromatography column through a peristaltic pump. Coll...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com