Method for preparing 7-phenylacetyl amino-3-allyl-4-cefazolin acid p-methoxybenzyl acetate
A technology of p-methoxybenzyl ester and phenylacetamide, which is applied in the field of preparation of cephalosporin pharmaceutical intermediates, can solve the problems of high manufacturing cost, large toxic and side effects, high production cost, etc., to reduce the cost of raw materials and speed up the reaction speed , the effect of reducing production costs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] (1) Mix 30.75g (0.063mol) of compound Ⅰ 7-phenylacetamido-3-chloromethyl-4-cephenoic acid p-methoxybenzyl ester, 8.0g (0.077mol) of sodium bromide, 2.04g (0.012 mol) Potassium iodide, 19.8g (0.075mol) triphenylphosphine were successively added to the mixed solution of 185mL methylene chloride and 125mL water, the pH of the reaction process was controlled by adding 2mol / L hydrochloric acid to be 2-3, stirred evenly, and heated to reflux After reacting for 2 hours, compound III was obtained, and the reaction progress was monitored by HPLC (when the compound I decreased below 0.5%, the reaction was terminated).
[0027] (2) After 2 hours, the reaction is basically completed, and the layers are statically separated. The temperature of the organic layer and the dichloromethane layer is lowered to 0-5°C, and 100mL of sodium hydroxide aqueous solution with a mass concentration of 3% is added dropwise, and the temperature is controlled at 5-10°C. The addition was controlled wit...
Embodiment 2
[0030]After the reaction in step (2) of Example 1 is completed, add 25 mL of water, 185 mL of dichloromethane, 0.8 g of sodium bromide, and 0.2 g of potassium iodide to the aqueous layer of step (2) of separation and recovery, and then add 30.75 g (0.063 mol ) p-methoxybenzyl 7-phenylacetamido-3-chloromethyl-4-cephemate, 19.8 g (0.075 mol) of triphenylphosphine. The pH of the reaction process is controlled to be 2-3 by adding 2 mol / L hydrochloric acid, and the mixture is stirred evenly; the temperature is raised to reflux for 2 hours, and the reaction process is monitored by HPLC. After 2 hours, the reaction was basically completed, and the layers were static and separated, and the organic phase was cooled to 0-5 ° C. The subsequent operations were carried out according to the method of Example 1, and 24.5 g of compound V 7-phenylacetamido-3-propene was obtained as a white solid. The yield of p-methoxybenzyl-4-cephemic acid was 81.4%, the purity was 97.3%, and the Z / E ratio wa...
Embodiment 3
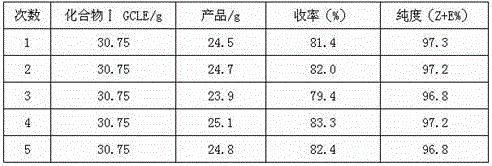
[0032] According to the feeding ratio and operation method of Examples 1 and 2, the process of step (2) water layer application is investigated respectively. Add a small amount of activated carbon to decolorize and filter the water layer obtained in step (2) each time, and apply it 5 times. The experimental results are shown in the table below.
[0033]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com