Preparation method of 3-methylol cefazolin
A technology of hydroxymethyl cefazolin and methyl morpholine, applied in the field of chemical drug synthesis, can solve the problems of unfavorable impurity research, low content of 3-hydroxymethyl cefazolin, inconvenient separation, etc., to reduce solvent residue, Improve product conversion rate and ensure the effect of activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
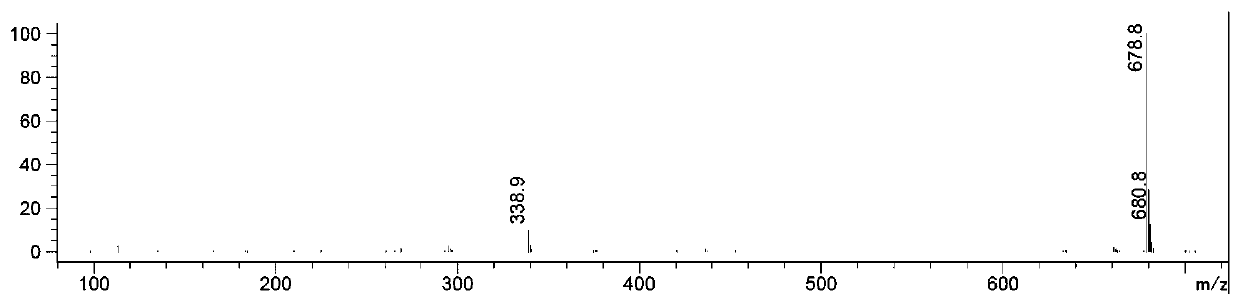
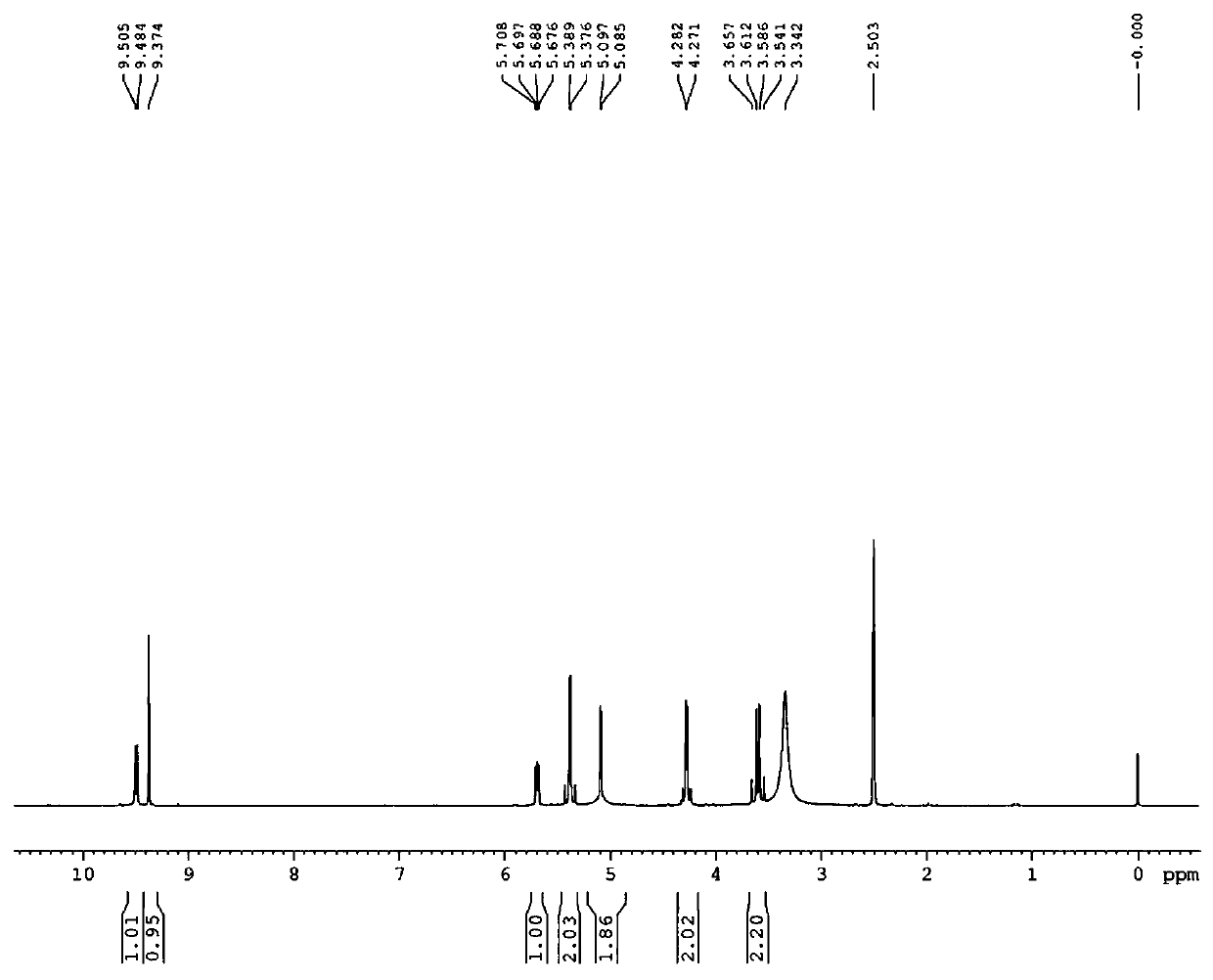
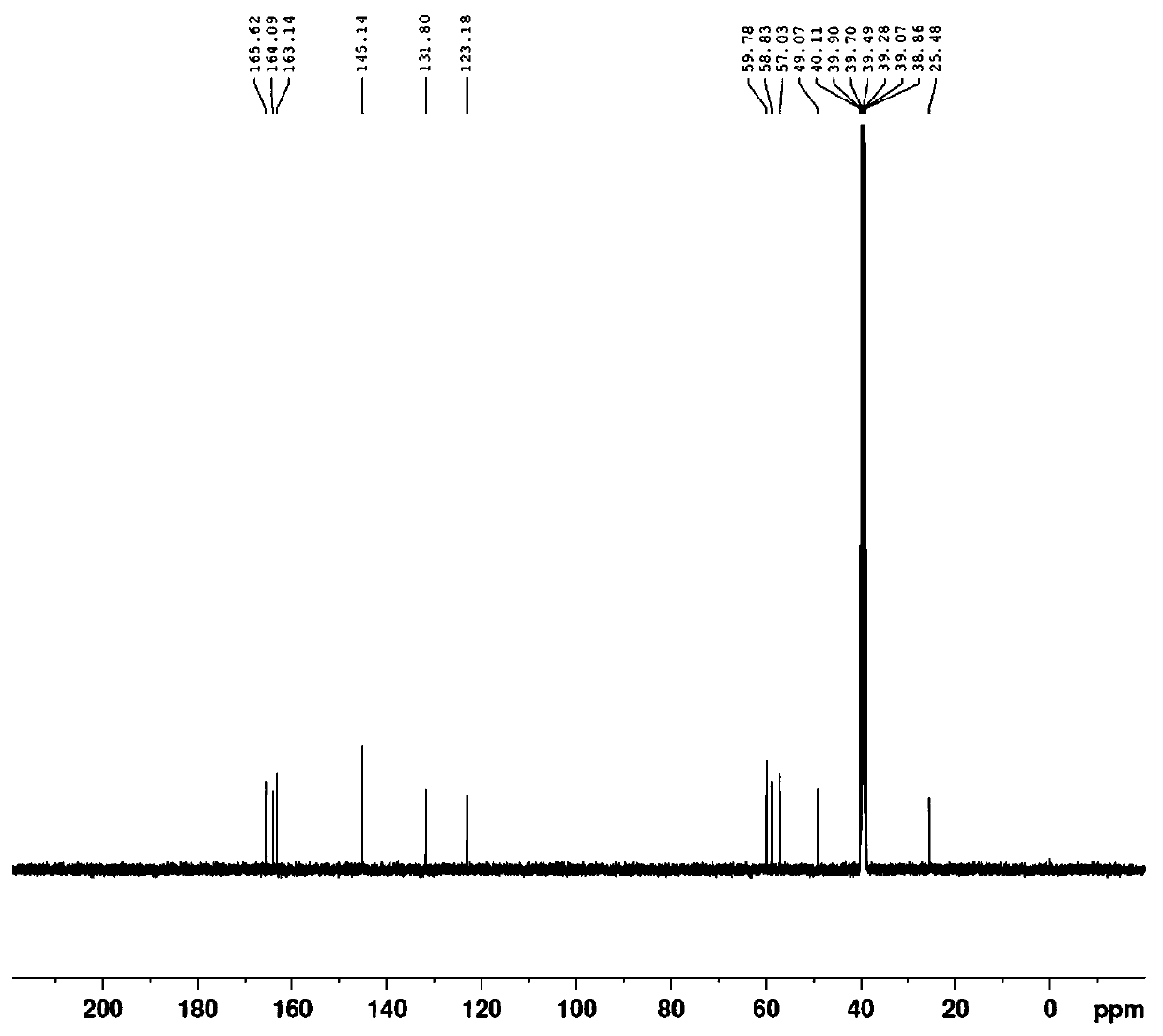
Image
Examples
Embodiment 1
[0027] a. Preparation of 7-ACA solution: Add 250ml chloroform and 35.4g 7-ACA (molecular weight 272.3, 0.13mol) to a 500ml four-necked flask under the condition of -20~-40℃, and then drop after temperature control at -15~20℃ Add 23.0g of tetramethylguanidine (molecular weight: 115.18, 0.20mol) until the 7-ACA solution is obtained after the solution is clear, and then the temperature is lowered to -25°C for use;
[0028] b. Preparation of tetrazolium acetic acid mixed anhydride solution: under the condition of -20~-60℃, add 280ml chloroform, 17.5g tetrazolium acetic acid (molecular weight 128.09, 0.14mol) and 19.3gN in another 1L four-necked flask. , After N-dimethylacetamide (molecular weight 87.12, 0.22mol), control the temperature at -20~-25℃, stir for 10min, and add 14.0g triethylamine (molecular weight 101, 0.14mol) dropwise in about 15min. After the tetrazolium acetic acid is dissolved, add 0.4g pyridine and 19.5g pivaloyl chloride (molecular weight 155.0, 0.13mol) successiv...
Embodiment 2
[0035] a. Preparation of 7-ACA solution: Add 400ml of dichloromethane and 35.4g of 7-ACA to a 500ml four-necked flask at -20~-40℃, add tetramethylguanidine dropwise after controlling the temperature at -15~20℃ 20.0g, until the 7-ACA solution is obtained after the solution is clarified, then the temperature is lowered to -25°C for use
[0036] b. Preparation of tetrazolium acetic acid mixed anhydride solution: under the condition of -20~-60℃, add 350ml dichloromethane, 21.78g tetrazolium acetic acid (0.17mol) and 26.5gN in another 1L four-neck flask. After N-dimethylacetamide (0.30mol), control the temperature at -20~-25℃ and stir for 10min, then add 21.5g triethylamine (0.20mol) dropwise for about 15min. When the tetrazolium acetic acid is dissolved, Then add 1.74g 2,6-lutidine and 21.7g pivaloyl chloride (0.14mol) successively, control the temperature at -20~-28℃ and stir for 1.5h to obtain the tetrazolium acetic acid mixed anhydride solution;
[0037] c. Preparation of 3-hydroxy...
Embodiment 3
[0043] a. Preparation of 7-ACA solution: Add 320ml of dichloromethane and 35.4g of 7-ACA in a 500ml four-necked flask at -20~-40℃, add tetramethylguanidine dropwise after controlling the temperature at -15~20℃ 18.1g, until the 7-ACA solution is obtained after the solution is clarified, then the temperature is lowered to -25°C for use;
[0044] b. Preparation of tetrazolium acetic acid mixed anhydride solution: Add 450ml dichloromethane, 25.0g tetrazolium acetic acid and 28g N,N-dimethyl ethyl into another 1L four-neck flask at -20~-60℃ After amide, after stirring at -20~-25℃ for 10min, 22.5g triethylamine was added dropwise in about 15min. When the tetrazolium acetic acid is dissolved, then 2.0g N-methylmorpholine and 23.25g special Valeryl chloride, temperature-controlled -20~-28℃, stirred for 1.5h to obtain tetrazolium acetic acid mixed anhydride solution;
[0045] c. Preparation of 3-hydroxymethyl cefazolin:
[0046] 1. Transfer the 7-ACA solution to the tetrazolium acetic acid ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com