Novel crystal form of cefazolin sodium and preparation method thereof
A technology of cefazolin sodium and crystal form, which is applied in the field of new crystal form of cefazolin sodium and its preparation, can solve the problems of relatively high requirements for drying and storage conditions, irregular product appearance, easy dehydration or moisture absorption, etc. It can achieve the effects of drying and long-term storage, good thermal stability and good stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
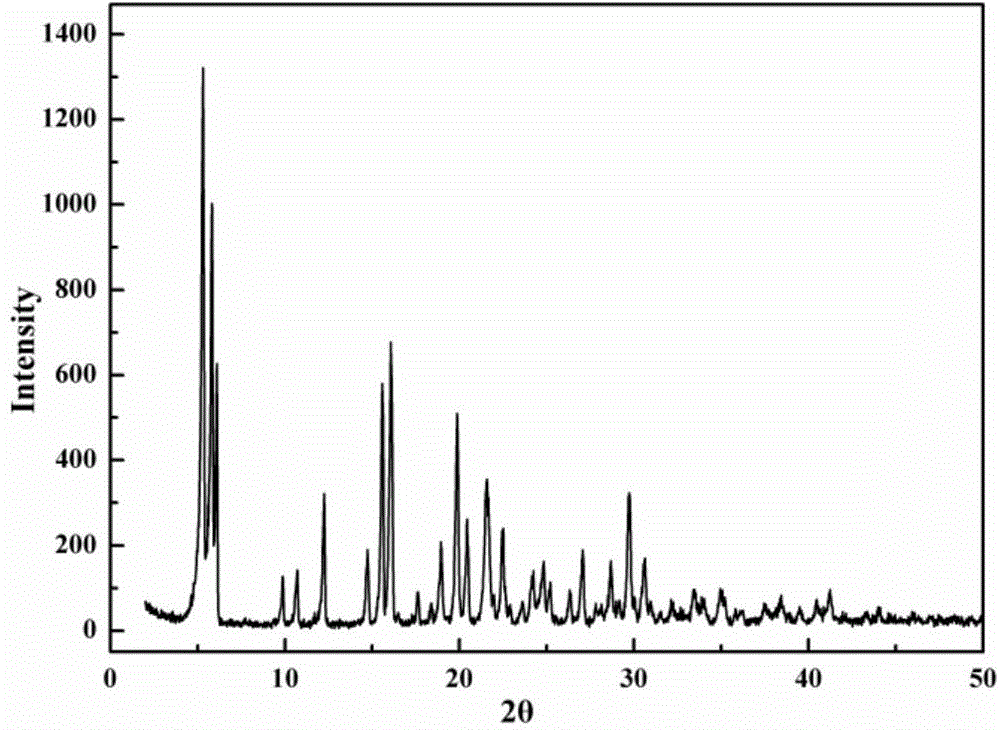
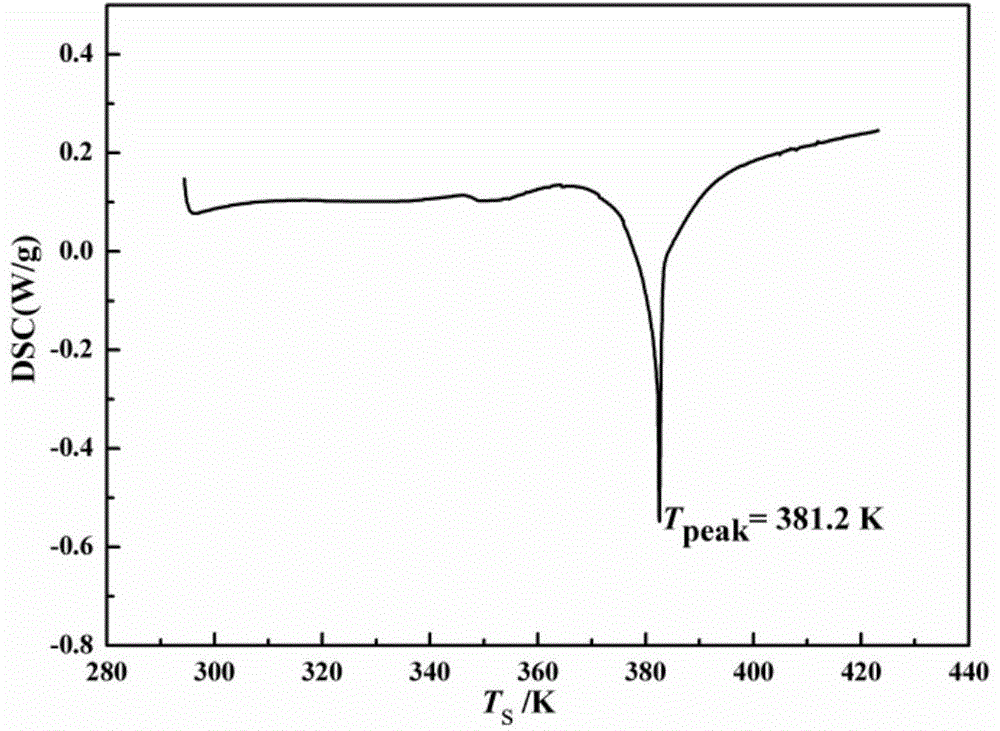
[0026] Add 30g of cefazolin sodium product into 100ml of N,N-dimethylacetamide to form a suspension of 0.3g / ml, keep the stirring speed at 200r / min, and raise the temperature of the suspension at a rate of 0.1°C / min to 30°C, stir at constant temperature for 0.5h, then programmatically cool down to 0°C at a rate of 1°C / min, and grow crystals at constant temperature for 0.5h, filter the obtained crystal slurry, and place the obtained wet crystal product at 20°C under vacuum After drying at 0.1Mpa for 20 hours, the purity of the obtained new crystalline form of cefazolin sodium is 99.6%, and the process yield is 90.0%. Its X-ray powder diffraction pattern is as figure 2 As shown, there are characteristic peaks at diffraction angles 2θ=5.2, 5.6, 10.6, 14.7, 15.5, 16.1, 19.8, 20.4, 22.4, 26.9, 28.6, and 29.6 degrees; its DSC analysis results are as follows image 3 As shown, there is an obvious endothermic peak at 381.5K, and its thermal stability is better, which is more conduci...
Embodiment 2
[0028] Add 30g of cefazolin sodium product into 60ml of N,N-dimethylformamide to form a 0.5g / ml suspension, keep the stirring speed at 400r / min, and heat the suspension to 35°C at a rate of 0.8°C / min. ℃, stirring at constant temperature for 1.5h, then cooling down to 15°C at a rate of 0.5°C / min, and growing crystals at 15°C for 2h, filtering the resulting suspension, and placing the obtained wet crystal product at 65°C under a vacuum of After drying at 0.09Mpa for 2 hours, the purity of the new crystalline form of cefazolin sodium obtained was 99.5%, and the process yield was 91.2%. The X-ray powder diffraction pattern of the new crystal form has characteristic peaks at diffraction angles 2θ=5.0, 5.4, 10.8, 14.6, 15.5, 16.1, 19.7, 20.5, 22.6, 26.9, 28.8, and 29.8 degrees; the new crystal form product is analyzed by DSC The results show that there is an endothermic peak at 379.2K, which has better thermal stability and is more conducive to drying and long-term storage; the appe...
Embodiment 3
[0030]Add 30 g of cefazolin sodium product into 50 ml of methanol to form a suspension of 0.6 g / ml, keep the stirring speed at 600 r / min, heat the suspension to 45 °C at a rate of 1 °C / min, stir at constant temperature for 8 h, and then Cool down to 20°C at a speed of 0.1°C / min, and grow crystals at 20°C for 1 hour at a constant temperature, filter the resulting suspension, and dry the obtained wet crystal product at 35°C and a vacuum of 0.1Mpa for 5 hours to obtain The purity of the new crystalline form of cefazolin sodium is 99.6%, and the process yield is 95%. The X-ray powder diffraction pattern of the new crystal form has characteristic peaks at diffraction angles 2θ=5.0, 5.7, 10.7, 14.9, 15.4, 16.0, 19.8, 20.2, 22.2, 26.9, 28.5, and 29.8 degrees; the new crystal form product is analyzed by DSC The results show that there is an endothermic peak at 380.1K, and its thermal stability is better, which is more conducive to drying and long-term storage; the appearance of the ne...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com