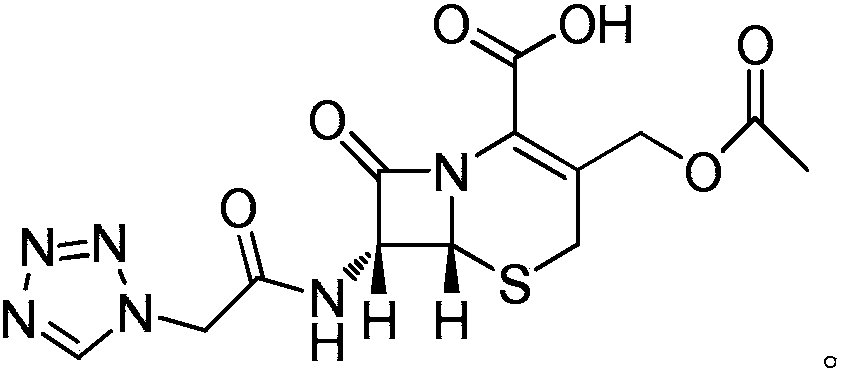
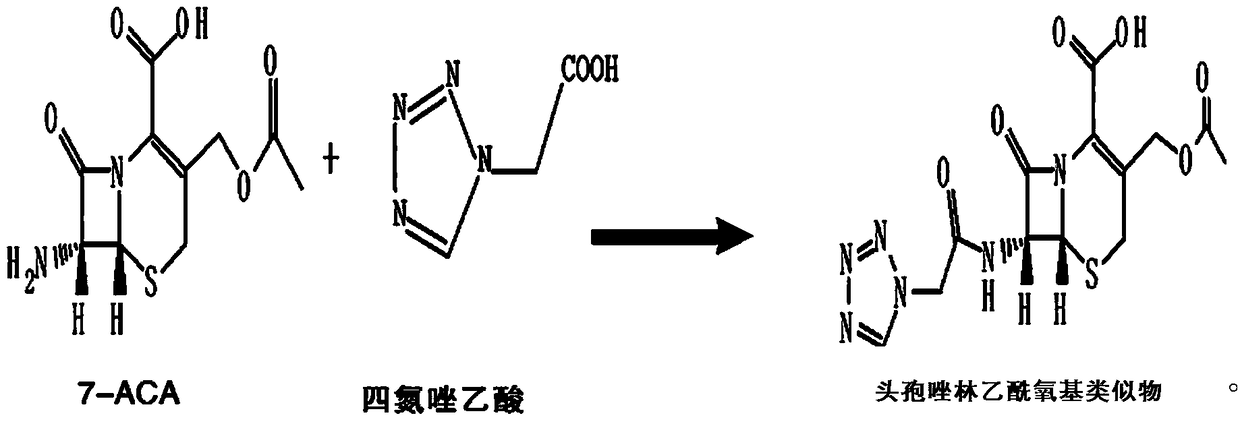
Cefazolin acetoxy analogue preparation method
A technology for cefazolin acetoxy analogs, which is applied in the field of preparation of cefazolin acetoxy analogs, can solve problems such as undiscovered, and achieve the effects of easy separation and purification, and simple and quick synthesis operations
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] Add 20.05g of 7-ACA and 150mL of dichloromethane into a 500mL four-neck flask, stir for 15min, control the temperature to -5°C~-10°C, add 9.8mL of tetramethylguanidine dropwise, and the dropwise addition is completed in about 15min, and continue to stir for 30min until the 7-ACA is completely dissolved, cool down to -30°C for later use; add 150mL of dichloromethane, 23.52g of tetrazoleacetic acid, 40.2mL of N,N-dimethylacetamide into another 500mL four-necked bottle, and control the temperature at -10 ℃~-15℃, stir for 15min, add 10mL triethylamine dropwise in about 20min, stir for 15min after adding, add 10mL pivaloyl chloride dropwise in about 30min, stir for 2h; Add dropwise into tetrazolium acetic acid mixed anhydride, stir for 1 hour, then control the temperature at 10-35°C, add 200mL of water, adjust the pH to 6.0-6.5 with triethylamine, stir and hydrolyze for 15 minutes, collect the water phase, decolorize with activated carbon, and filter , add 10% hydrochloric a...
Embodiment 2
[0032] Add 20.03g of 7-ACA and 150mL of dichloroethane into a 500mL four-neck flask, stir for 15 minutes, control the temperature to -10°C to -25°C, add 10.1mL of tetramethylguanidine dropwise, and continue stirring after about 15 minutes. 30min until 7-ACA is completely dissolved, cool down to -30°C for later use; add 150mL dichloroethane, 23.69g tetrazoleacetic acid, 41.5mL N,N-dimethylacetamide to another 500mL four-necked bottle, and control the temperature -20℃~-35℃, stir for 15min, add 10mL triethylamine dropwise in about 20min, stir for 15min after the addition, add 10mL pivaloyl chloride dropwise in about 30min, stir for 2h; control temperature <-30℃, add 7-ACA Add the dissolved solution dropwise to tetrazole acetic acid mixed anhydride, stir and react for 1h, then control the temperature at 10-35°C, add 200mL of water, adjust the pH to 7.0-7.5 with triethylamine, stir and hydrolyze for 15min, collect the water phase, and decolorize it with activated carbon , filter, a...
Embodiment 3
[0034]Add 20.12g of 7-ACA and 150mL of toluene into a 500mL four-neck flask, stir for 15min, control the temperature to -25°C~-35°C, add 12.4mL of tetramethylguanidine dropwise, and finish adding in about 15min, continue stirring for 30min until Dissolve all 7-ACA, cool down to -30°C for later use; add 150mL toluene, 23.16g tetrazolium acetic acid, 40.8mL N,N-dimethylacetamide to another 500mL four-necked bottle, and control the temperature at -35°C to -40°C ℃, stir for 15min, add 10mL triethylamine dropwise for about 20min, stir for 15min after adding, add 10mL pivaloyl chloride dropwise for about 30min, stir for 2h; In azole acetic acid mixed anhydride, stir and react for 1h, then control the temperature at 10-35°C, add 200mL of water, adjust the pH to 8.0-8.5 with triethylamine, stir and hydrolyze for 15min, collect the water phase, decolorize with activated carbon, filter, add 10% Adjust the pH to 1.5-2.5 with hydrochloric acid, then reduce the temperature of the system to...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com