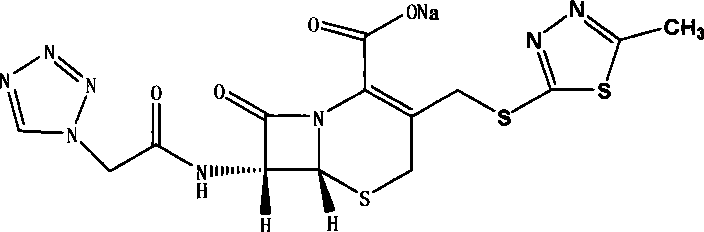
Preparation of cefazolin sodium sterilized raw medicine
A technology for cefazolin sodium and bulk drug, which is applied in the field of preparation of cefazolin sodium aseptic bulk drug, can solve the problems of high, difficult to control substances and the like, and achieves the effects of simple method, easy operation and stable quality
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
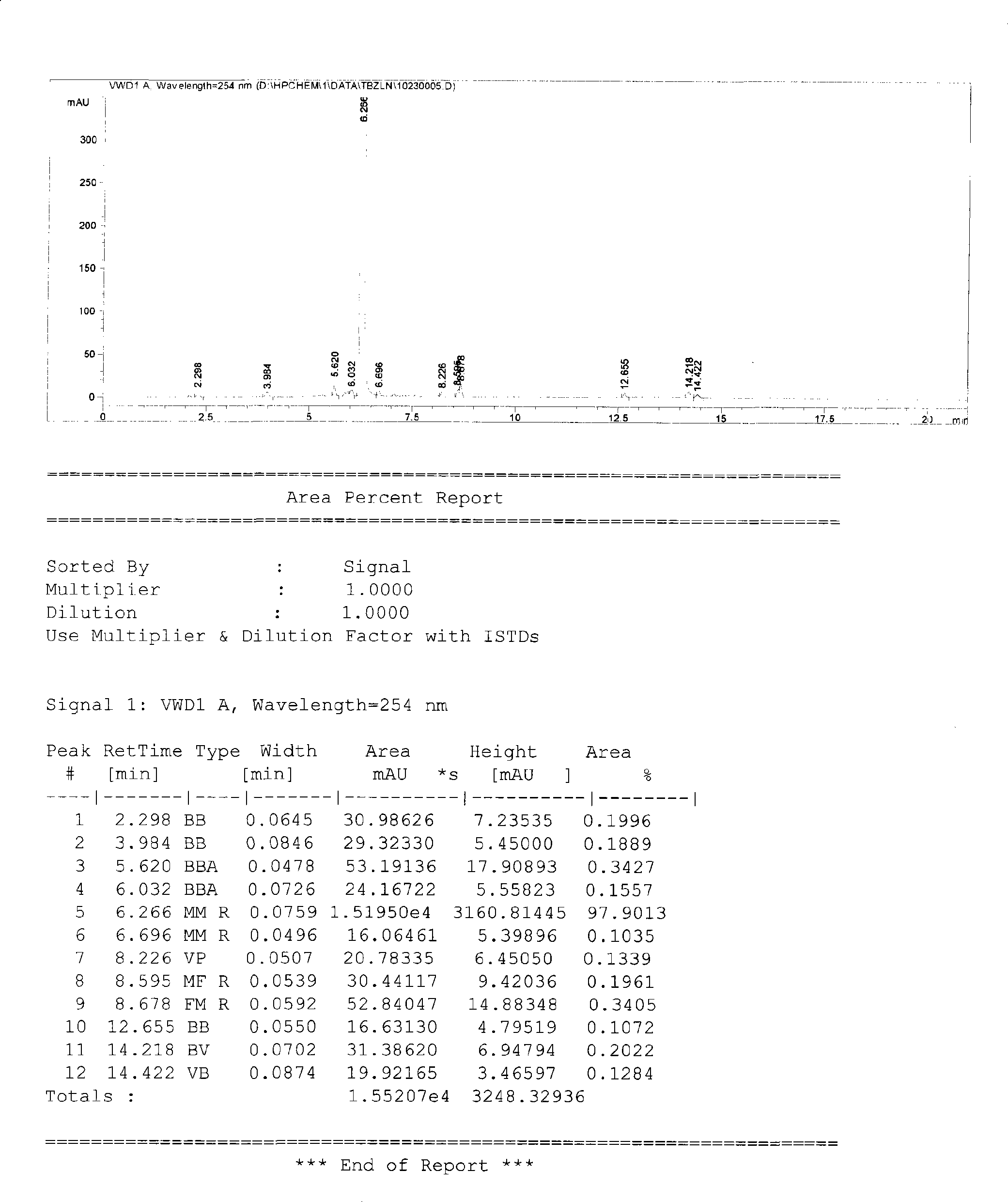
[0023] Suspend 5.0 g of cefazolin in 50 ml of deionized water, and neutralize with saturated sodium bicarbonate solution to pH = 4.5 in a stirred, airtight container with a water bath at a constant temperature of 45°C and under reduced pressure (the water pump pressure gauge indicates 0.01Mpa). , the solution is clear and transparent, add 0.1 g of activated carbon and continue to stir for 15 minutes to decolorize. After the filter cloth is filtered to remove the activated carbon, the filtrate is then filtered with a 0.22 μm microporous filter membrane. Control the thickness of the solution to 1.0cm, freeze the product to -30°C, keep it for 1 hour, pump a vacuum, keep the vacuum at 0-10Pa, raise the temperature to 40°C, keep it for 5 hours, and the freeze-drying is completed. Out of the box, pulverized, and aseptically packaged to obtain cefazolin sodium 5.2g. Yield 99.0%, (theoretical value 5.25g), moisture is 0.9%, content is 99.5% in terms of dry product; Related substances ...
Embodiment 2
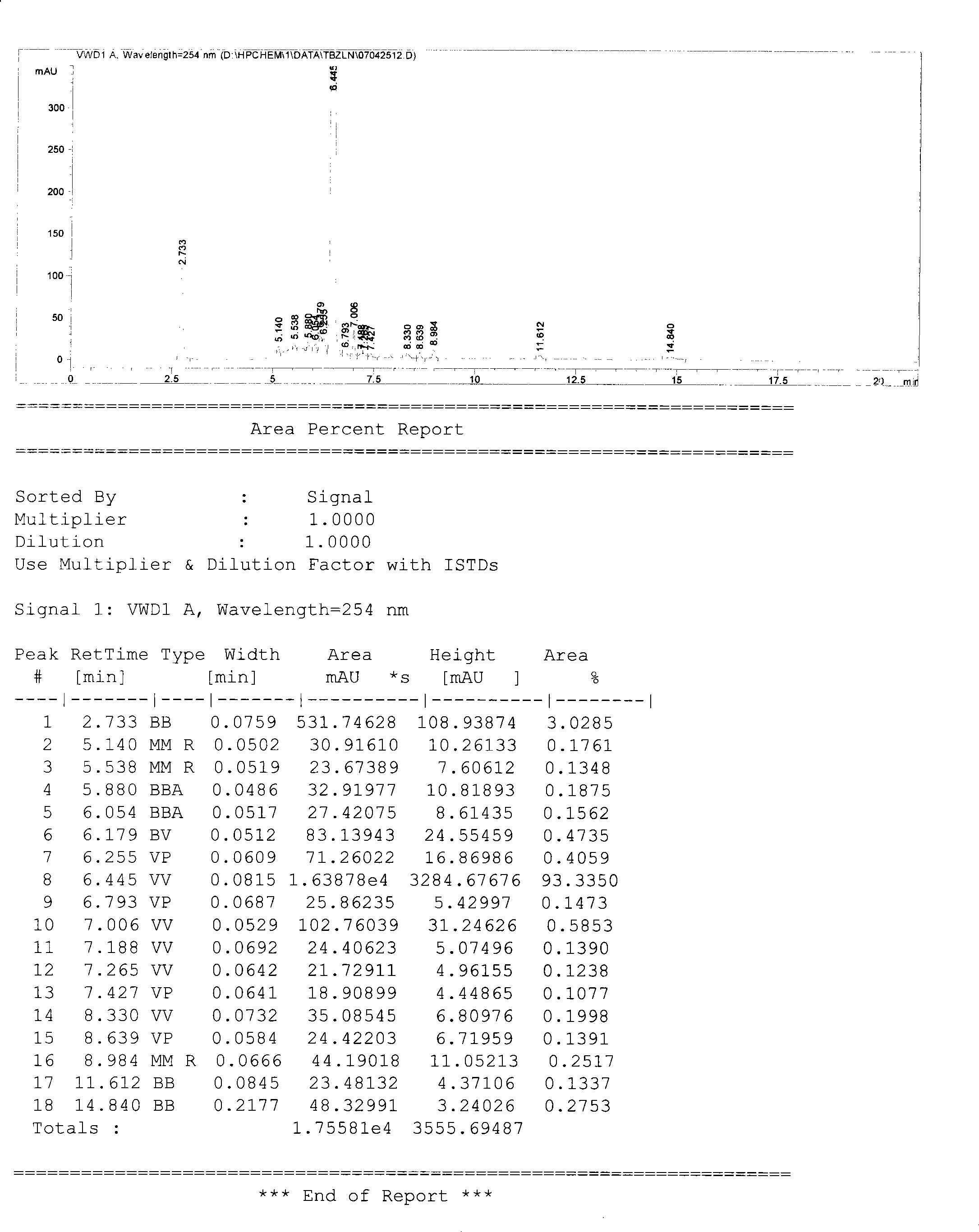
[0025] 2500g of cefazolin was neutralized to pH=5.4 with 8% sodium bicarbonate solution in a water bath with constant temperature of 35°C and reduced pressure (indicating 0.082Mpa on the water pump pressure gauge) in a stirred and airtight container. The solution was clear and transparent, and the constant temperature was continued. After reacting for 30 minutes, the reaction solution was filtered with a titanium rod in a 10,000-class clean area to remove activated carbon, and the filtrate was filtered with a 0.22 μm microporous membrane in a local 100-class aseptic area, and the obtained filtrate was poured into a freeze-drying tray, and the thickness of the solution was controlled to be 2.0cm, freeze the product to -40°C, keep it for 3 hours, vacuumize, keep the vacuum at 20-35Pa, then raise the temperature to 50°C, keep it for 5 hours, then keep the vacuum at 0-10Pa for 4 hours, and the freeze-drying is over . Out of the box, pulverized, and aseptically packaged to obtain 2...
Embodiment 3
[0027] 2500g of cefazolin was neutralized to pH=8.0 with 1% sodium hydroxide solution under constant temperature of water bath at 30°C and reduced pressure (indicating 0.062Mpa on the water pump pressure gauge) in a stirred and airtight container, the solution was clear and transparent, and activated carbon was added 65g continued to stir and decolorize for 120min. After the reaction liquid was filtered with a titanium rod to remove the activated carbon in a 10,000-class clean area, the filtrate was filtered with a 0.22μm microporous membrane in a local 100-class aseptic area, and the obtained filtrate was poured into a freeze-drying tray. Control the thickness of the solution to 1.5cm, freeze the product to -35°C, keep it for 9 hours, then maintain the vacuum at 0-10Pa for 3 hours, then pump the vacuum and maintain the vacuum at 20-35Pa for sublimation, then raise the temperature to 50°C, keep After 12 hours, freeze-drying was completed. Out of the box, pulverized, and asepti...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com