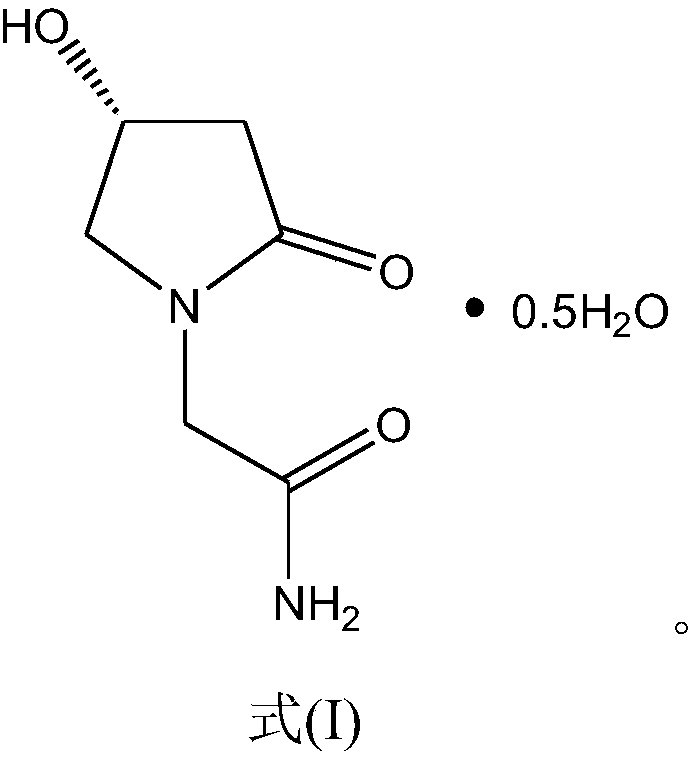
Freeze-dried composition containing dextrorotation oxiracetam 1/2 aquo-complex and preparation method of freeze-dried composition
A composition and freeze-drying technology, applied in the directions of drug combinations, medical preparations containing active ingredients, freeze-dried delivery, etc., can solve the problems of short validity period of drugs, accelerated water degradation, and increase of related substances, and achieve long freeze-drying time. , The effect of smooth water vapor circulation and low moisture absorption
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] Dexoxiracetam was dissolved in a mixed solvent (volume ratio isopropanol:ethyl acetate=1:1), wherein the mass volume ratio (g / mL) of dexoxiracetam to the mixed solvent was 1: 5. Filtrate, cover and seal the filtrate, stir at a speed of 100-150r / min for 15h, filter again, after filtration, let the filtrate stand in a desiccator to evaporate the solvent to form crystals, collect the crystals, and store the collected crystals at 25°C, relatively The humidity is 70-75% under the condition of drying 4h, vested.
[0028] Elemental analysis, determined that the crystal molecular formula is C 6 h 10 N 2 o 3 0.5H 2 O. The moisture in the Dexoxiracetam compound of the present invention was measured by Karl Fischer method to be 5.53% (theory: 5.52%).
[0029] Differential scanning thermal analysis (DSC) chart determination:
[0030]Test equipment and conditions: DSC test adopts NETZSCH DSC200PC tester. The test method is to accurately weigh a certain amount (1 ~ 2mg) of the...
Embodiment 2
[0032] Dissolve dexoxiracetam in a mixed solvent (volume ratio isopropanol:petroleum ether=1:3), wherein the mass volume ratio (g / mL) of dexoxiracetam to the mixed solvent is 1:3 , filter, seal the filtrate with a cover, stir at a speed of 100-150r / min for 20h, and filter again. After filtration, the filtrate is left in a desiccator to evaporate the solvent to form crystals, collect the crystals, and store the collected crystals at 20 ° C, relative humidity Drying for 6h under the condition of 60-65%, vested.
[0033] Elemental analysis, determined that the crystal molecular formula is C 6 h 10 N 2 o 3 0.5H 2 O. The moisture in the Dexoxiracetam compound of the present invention was measured to be 5.58% (theory: 5.52%) by the Karl Fischer method.
Embodiment 3
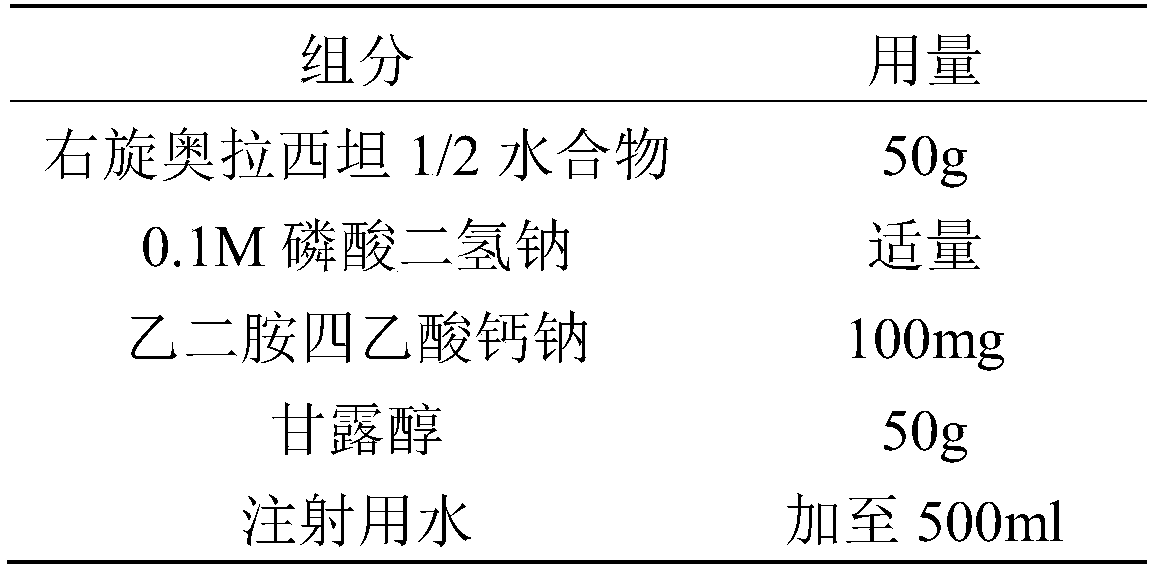
[0036] prescription
[0037]
[0038] Preparation:
[0039] Take 400ml of boiled water for injection, add the prescribed amount of calcium sodium edetate and mannitol, stir to dissolve, add the prescribed amount of Dexoxiracetam 1 / 2 hydrate and stir to dissolve, and add 0.1M diphosphate diphosphate Adjust the pH value of the sodium hydrogen solution to 5.0, add 0.2% activated carbon for needles according to the prepared amount, heat the liquid medicine to about 55°C, stir for 30 minutes, filter and decarbonize, add water for injection to the total amount, and then use a 0.22 μm microporous filter membrane Fine filtration, filling, half stoppering.
[0040] Freeze-drying process:
[0041] Pre-freezing stage: lower the temperature of the shelf to -45°C, quickly put the product in, and when the temperature of the product reaches -35±2°C, continue to keep warm for 4 hours, and keep the vacuum in the box at 10±2Pa;
[0042] Primary drying stage: keep the vacuum degree in the ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com