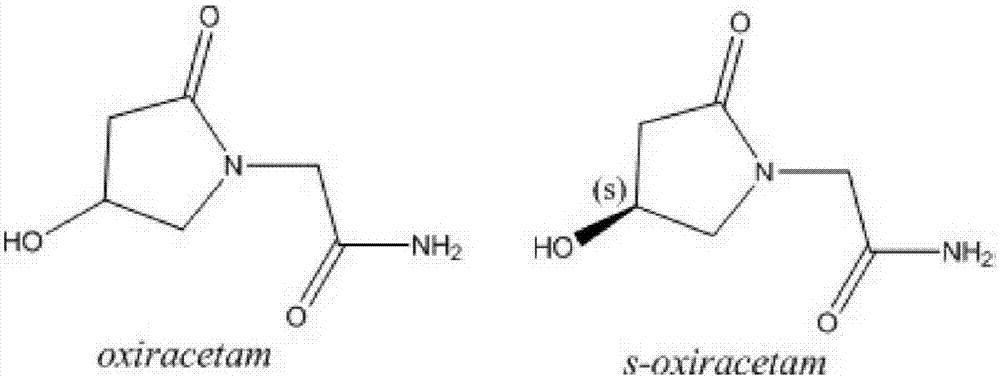
Levo oxiracetam for injection and preparation method thereof
A technology for injection and water for injection, which is applied in the field of levoxiracetam for injection and its preparation, which can solve the problems of large pH changes of the solution, poor clarity of the finished product, and many impurities, and achieve less impurities and good clarity of the product , good stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
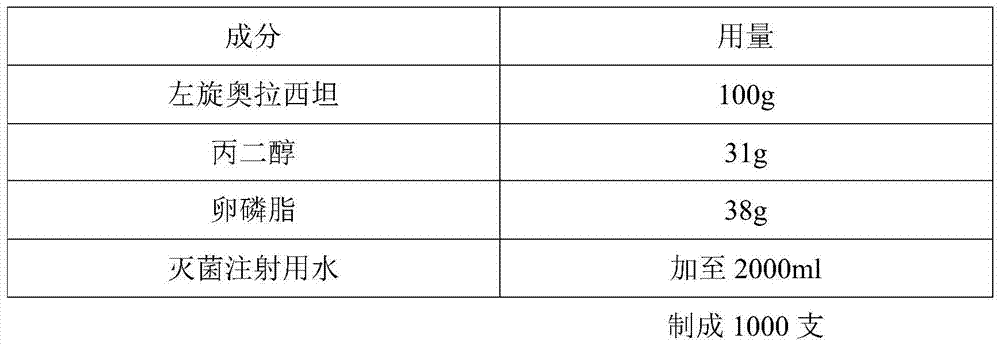
[0022] A kind of levoxiracetam for injection, prepared according to the following steps:
[0023]
[0024] Preparation process:
[0025] 1. Concentrated formulation: Add the above-mentioned raw and auxiliary materials into the batching tank, then add 2 / 3 of the prescription amount of sterile water for injection, stir, dissolve, and obtain a concentrated formulation;
[0026] 2. Dilute preparation: Take the concentrated preparation, add sodium phosphate buffer solution (precisely weigh 65.697g of disodium hydrogen phosphate and 2.346g of sodium dihydrogen phosphate, put it in a 1000ml volumetric flask, add purified water to dissolve, dilute to the scale , to obtain), adjust the pH to 6.5, add chitosan with a total volume of 0.3% to 0.5% (g / ml) to the above solution, stir, mix well, let stand for 35 to 45min, and filter with a 0.8 μm filter membrane Add 0.1% to 0.3% (g / ml) of activated carbon in the total volume, adsorb and decolorize, filter with a 0.45 μm filter membrane, ...
Embodiment 2
[0067] A kind of levoxiracetam for injection, prepared according to the following steps:
[0068]
[0069] Preparation process: prepared according to the preparation process of Example 1.
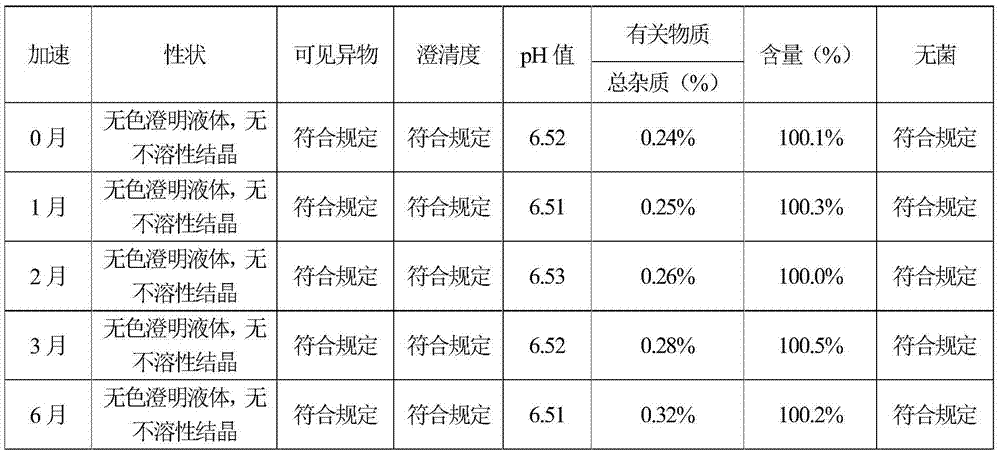
[0070] Press the test method of embodiment 1, carry out respectively stability test investigation, clarity contrast test and pH adjuster to the influence experiment of solution pH before and after product sterilization, stability test result shows that accelerates 6 months sample quality is stable, long-term 18 months The quality is stable, so this product is valid for at least 18 months; the test results of the clarity comparison test show that the clarity of the sample produced in Example 2 is less than No. 0.5 standard turbidity liquid, and the clarity of this product is good; different pH regulators can sterilize the product The experiment of the influence of solution pH before and after showed that the solution pH of the sample prepared in Example 2 did not change substantially befo...
Embodiment 3
[0072] A kind of levoxiracetam for injection, prepared according to the following steps:
[0073]
[0074] Preparation process: prepared according to the preparation process of Example 1.
[0075] Press the test method of embodiment 1, carry out respectively stability test investigation, clarity contrast test and pH adjuster to the influence experiment of solution pH before and after product sterilization, stability test result shows that accelerates 6 months sample quality is stable, long-term 18 months The quality is stable, so this product is valid for at least 18 months; the test results of the clarity contrast test show that the clarity of the sample produced in Example 3 is less than No. 0.5 standard turbidity liquid, and the clarity of this product is good; different pH regulators can sterilize the product The experiment on the influence of solution pH before and after showed that the solution pH of the sample prepared in Example 3 did not change substantially before...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com