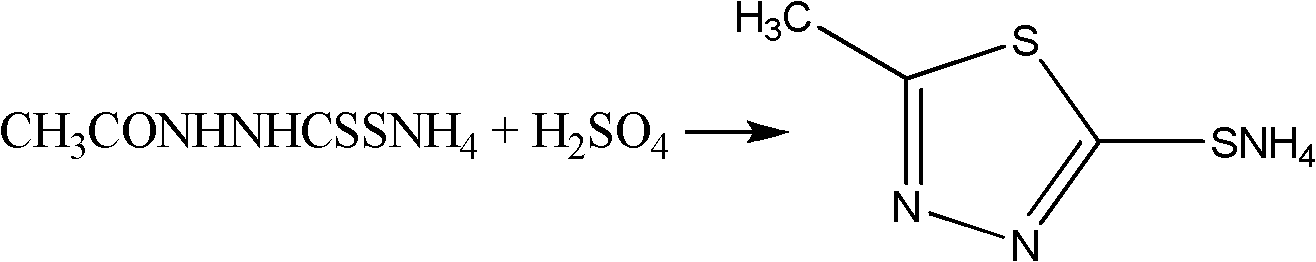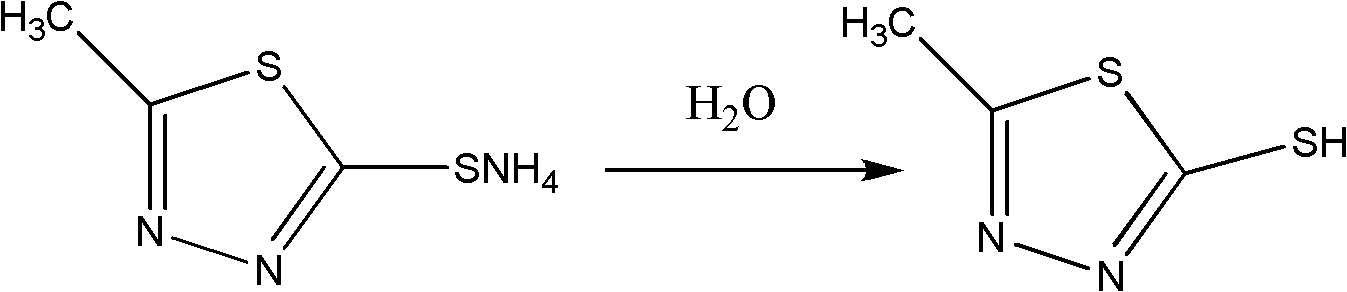Synthesis method of 2-methyl-5-sulfydryl-1,3,4-thiadiazole
A synthetic method, thiadiazole technology, applied in the field of medicine, can solve the problems of high potassium hydroxide content, high cost, troublesome drying process, etc., and achieve the effect of overcoming long production cycle, reducing raw material cost, and shortening production cycle
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0021] Take 112.0g of hydrazine hydrate with a mass percentage of 80%, and put 156.0g of ethyl acetate in a four-neck flask, stir, and heat to reflux for 8 hours. Below ℃, pour 370.0g of liquid ammonia, add 104.0ml of carbon disulfide dropwise, the dropping temperature does not exceed 40℃, react at a temperature of 35℃~45℃ for 4hrs, cool down to below 5℃, filter with suction, wash with ice ethanol, and obtain 242.0g Ammonium salt——N-acetylhydrazino ammonium dithioformate; take 512.0ml of concentrated sulfuric acid and cool it down to 0°C, then add the above-mentioned 242.0g of ammonium salt in batches, after the addition, stir for 2 hours to obtain feed liquid I; take 2500ml Ice water, slowly pour the material liquid I into it so that the temperature does not exceed 10 ° C, stir, the product precipitates, and filter to obtain 165.0 g of the crude product of thiadiazole; then the crude product is conventionally purified and vacuum-dried to obtain 2-formazol 145.0 g of 5-mercapt...
Embodiment 2
[0023] Take 112.0g of hydrazine hydrate with a mass percentage of 80%, and put 156.0g of ethyl acetate in a four-necked flask, stir, and heat to reflux for 8 hours. After the reflux is completed, cool down to 25°C, add 95.0g of methanol, and continue to cool down to below 0°C. Pass in 368.0g of liquid ammonia, add 105.0ml of carbon disulfide dropwise at 0°C to 38°C, after the dropwise addition, stir and react at 35°C to 45°C for 4 hours, cool down to 2°C, filter with suction, wash with cold methanol twice, Ammonium salt——243.5 g of ammonium N-acetylhydrazinodithioformate was obtained.
[0024] The remaining steps were the same as in Example 1 to obtain 146.8 g of finished 2-methyl-5-mercapto-1,3,4-thiadiazole.
Embodiment 3
[0026] The solvent used was 100.0 g of a mixture of methanol and ethanol in an equal volume ratio, and the rest of the raw materials and reaction conditions were the same as in Example 2 to obtain 242.8 g of ammonium salt—N-acetylhydrazinodithioformate.
[0027] The rest of the steps were the same as in Example 1 to obtain 146.2 g of finished 2-methyl-5-mercapto-1,3,4-thiadiazole.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com