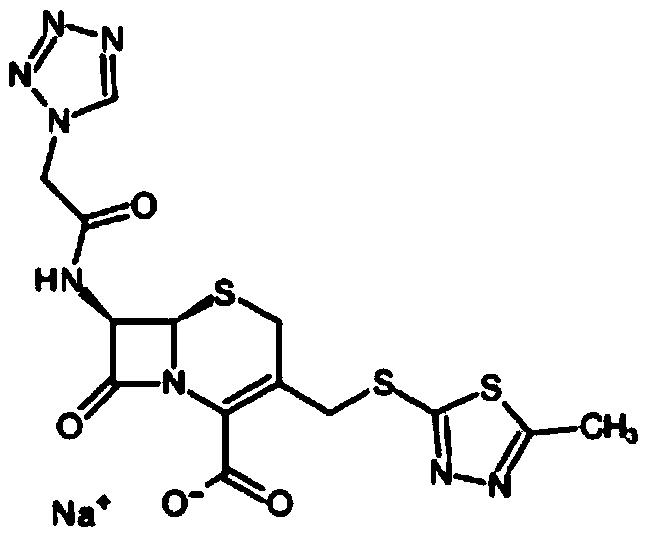
Purification method of cefazolin sodium
A technology of cefazolin sodium and a purification method, which is applied in the field of purification of cefazolin sodium, can solve the problems of degradation and new impurity generation, heavy environmental protection treatment burden, complicated operation and the like, and achieves reduction of waste water consumption, drug safety and high content Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] After the synthesis of cefazolin is completed, after adding water for hydrolysis, add 100ml of hydrolyzed solution containing 2g of cefazolin acid, add 50ml of dichloromethane, add 10% hydrochloric acid dropwise while stirring, control pH=1.3~1.6, and then let it stand for 10 minute. Separate the phases, discard the water phase, collect the dichloromethane phase, add 100ml of water, stir, adjust the pH to 6.4-6.7 with 10% sodium hydroxide solution, and let stand for 10 minutes. The phases were separated, and the dichloromethane phase was discarded to obtain a purified cefazolin sodium solution with a yield of 92.4%, a content of 98.1%, and a single compound of 0.21%. The purified cefazolin sodium solution is crystallized to obtain pure cefazolin sodium; the crystallization solvent is acetone; the volume of the crystallization solvent is 3 times the volume of the cefazolin sodium solution; and the crystallization temperature is 10-15°C.
Embodiment 2
[0030] After the synthesis of cefazolin is completed, after adding water for hydrolysis, add 100ml of hydrolyzed solution containing 2.5g of cefazolin acid, add 80ml of dichloromethane, add 12% hydrochloric acid dropwise while stirring, control the pH=1.3~1.6, and then let stand 10 minutes. Separate the phases, discard the water phase, collect the dichloromethane phase, add 150ml of water, stir, adjust the pH to 6.4-6.7 with 10% sodium hydroxide solution, and let stand for 10 minutes. The phases were separated, and the dichloromethane phase was discarded to obtain a purified cefazolin sodium solution with a yield of 93.0%, a content of 98.2%, and a single compound of 0.23%. The purified cefazolin sodium solution is crystallized to obtain pure cefazolin sodium; the crystallization solvent is acetone; the volume of the crystallization solvent is 4 times the volume of the cefazolin sodium solution; and the crystallization temperature is 10-15°C.
Embodiment 3
[0032] After the synthesis of cefazolin is completed, after adding water for hydrolysis, add 100ml of hydrolyzed solution containing 1.5g of cefazolin acid, add 100ml of dichloromethane, add 8% hydrochloric acid dropwise while stirring, control the pH=1.3~1.6, and then let stand 10 minutes. Separate the phases, discard the water phase, collect the dichloromethane phase, add 120ml of water, stir, adjust the pH to 6.4-6.7 with 8% sodium hydroxide solution, and let stand for 10 minutes. The phases were separated, and the dichloromethane phase was discarded to obtain a purified cefazolin sodium solution with a yield of 88%, a content of 98.6%, and a single compound of 0.15%. The purified cefazolin sodium solution is crystallized to obtain pure cefazolin sodium; the crystallization solvent is acetone; the volume of the crystallization solvent is 5 times the volume of the cefazolin sodium solution; and the crystallization temperature is 10-15°C.
[0033] The existing purification m...
PUM
| Property | Measurement | Unit |
|---|---|---|
| crystallization temperature | aaaaa | aaaaa |
| concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com