Preparation method of cefazolin sodium
A cefazolin sodium, condensation reaction technology, applied in the direction of organic chemistry, etc., to achieve the effect of less allergic phenomenon, reduce pollution, and improve quality
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
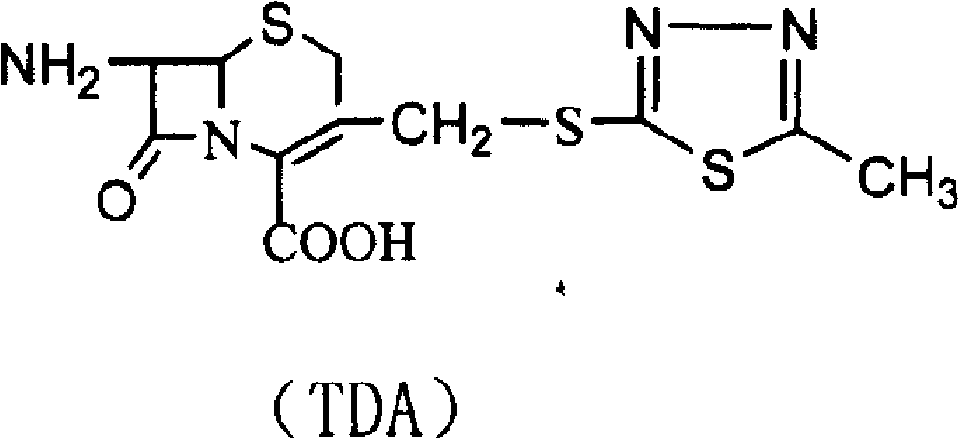
[0042] Step 1, three-position intermediate (TDA) of cefazolin sodium is synthesized
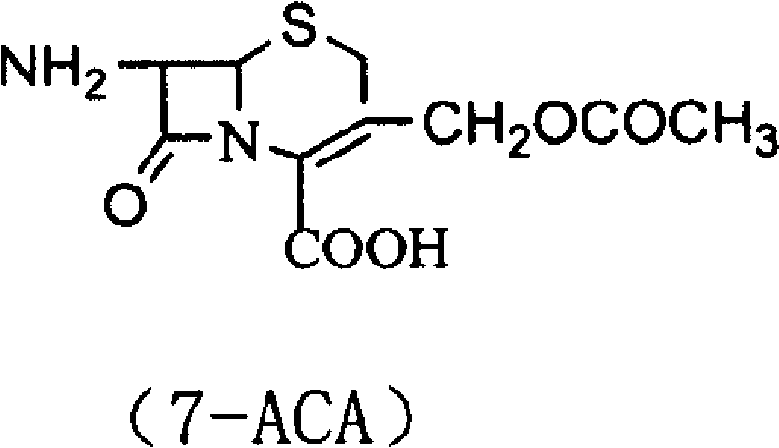
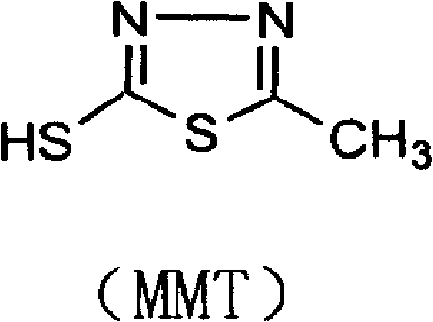
[0043] Add 500g of dimethyl carbonate and 500g of boron trifluoride-dimethyl carbonate solution into the condensation reaction bottle and stir, then put 150g of thiadiazole and 300g of 7-ACA into the reaction bottle, and raise the temperature at the same time, the reaction temperature is 35-40°C , reacted until the residue of 7-ACA was less than 1.0%. After the reaction is complete, transfer the reaction solution into a crystallization bottle containing 3000ml of deionized water and 500ml, add ammonia water dropwise to pH 2-4, cool down to 10°C, and stir for 1 hour to grow crystals. Filter, wash the material with acetone until the material liquid is colorless and drain, and put the wet product into the next step.
[0044] Step 2, cefazolin acid synthesis
[0045] Add 80g of tetrazolium acetic acid to 1000ml of dichloromethane, cool down to -50±1°C, add 120ml of triethylamine dropwise within...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com