Cefazolin sodium alkali degraded impurity compounds as well as preparation method and application thereof
A technology of cefazolin sodium base and azolin sodium base, which is applied in organic chemistry and other fields, and can solve problems such as affecting the efficacy of drugs, reducing drug safety, and not analyzing impurities in detail
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
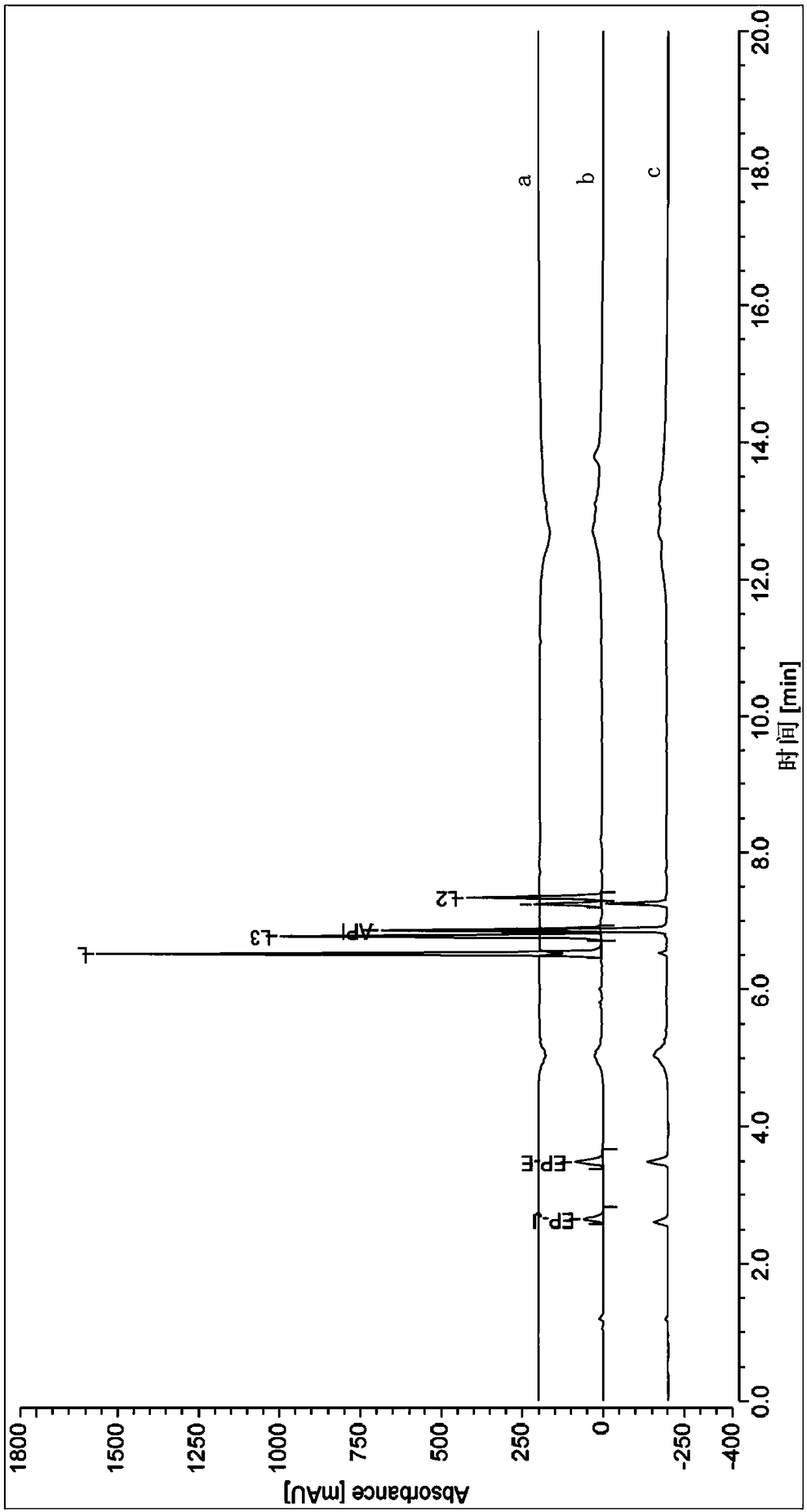
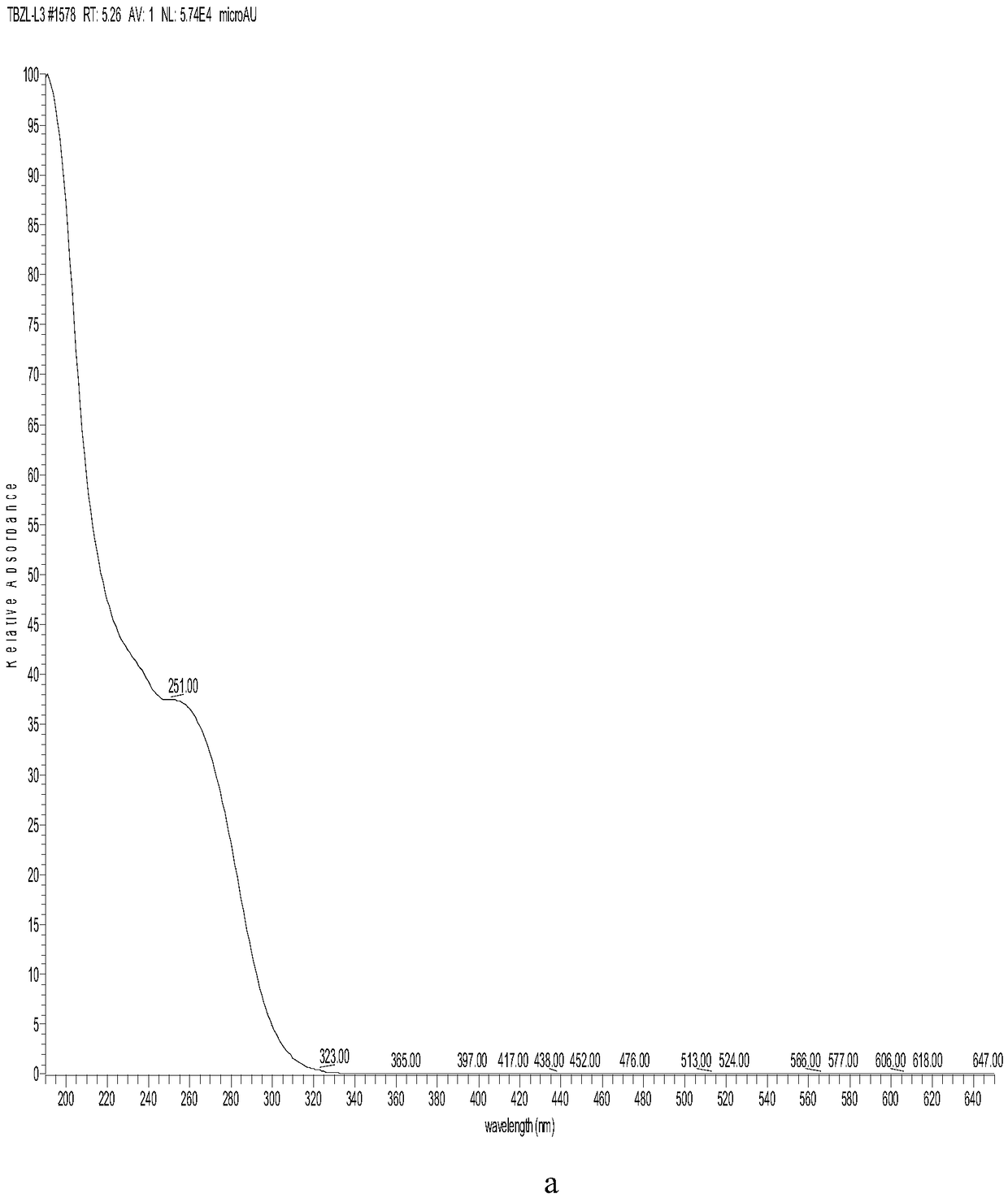
[0040] The embodiment of cefazolin sodium alkali degrading impurity compound represented by formula I in the present invention, the preparation method of the compound represented by formula I described in this embodiment comprises the following steps: adding cefazolin sodium alkali destruction solution into C18 column, Carry out gradient elution with 0.025mol / L potassium dihydrogen phosphate aqueous solution and the acetonitrile solution of 5% volume concentration, obtain the enrichment fraction of the compound shown in formula I, obtain the compound shown in formula I after 3 separations (note For impurity L).
[0041] The compound shown in formula I was washed several times with pure water, and then washed with acetonitrile solution with a volume concentration of 80%, to obtain the compound shown in formula I without salt, and the purity of the compound shown in formula I was 96.8%.
Embodiment 2
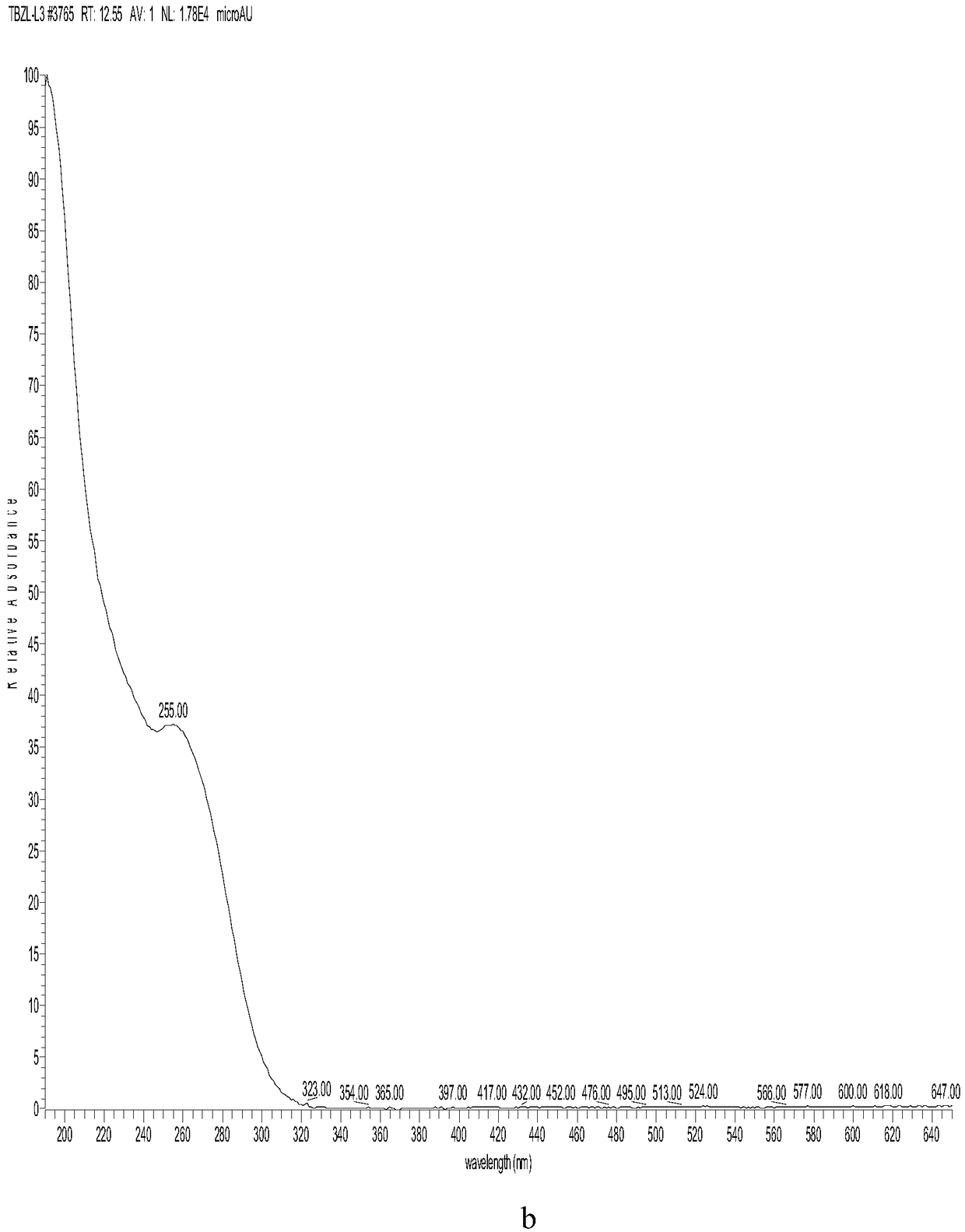
[0043] The embodiment of cefazolin sodium alkali degrading impurity compound represented by formula I in the present invention, the preparation method of the compound represented by formula I described in this embodiment comprises the following steps: adding cefazolin sodium alkali destruction solution into C18 column, Carry out gradient elution with the potassium dihydrogen phosphate aqueous solution of 0.05mol / L and the acetonitrile solution of 10% volume concentration, obtain the enrichment fraction of the compound shown in formula I, obtain the compound shown in formula I after 1 separation (note For impurity L).
[0044] The compound shown in formula I was washed several times with pure water, and then washed with acetonitrile solution with a volume concentration of 100%, to obtain the compound shown in formula I without salt, and the purity of the compound shown in formula I was 95.1%.
Embodiment 3
[0046]The embodiment of the preparation method of the cefazolin sodium alkali degraded impurity compound shown in the formula II of the present invention, the preparation method of the compound shown in the formula II described in this embodiment comprises the following steps: adding the cefazolin sodium alkali destruction solution to C18 In the column, gradient elution is carried out with an aqueous formic acid solution with a mass concentration of 0.05% and an acetonitrile solution with a volume concentration of 20%, to obtain an enriched fraction of the compound shown in the formula II, and the compound shown in the formula II is obtained after three separations ( Denoted as impurity L2), the purity of the compound shown in the obtained formula II was 96.2%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com