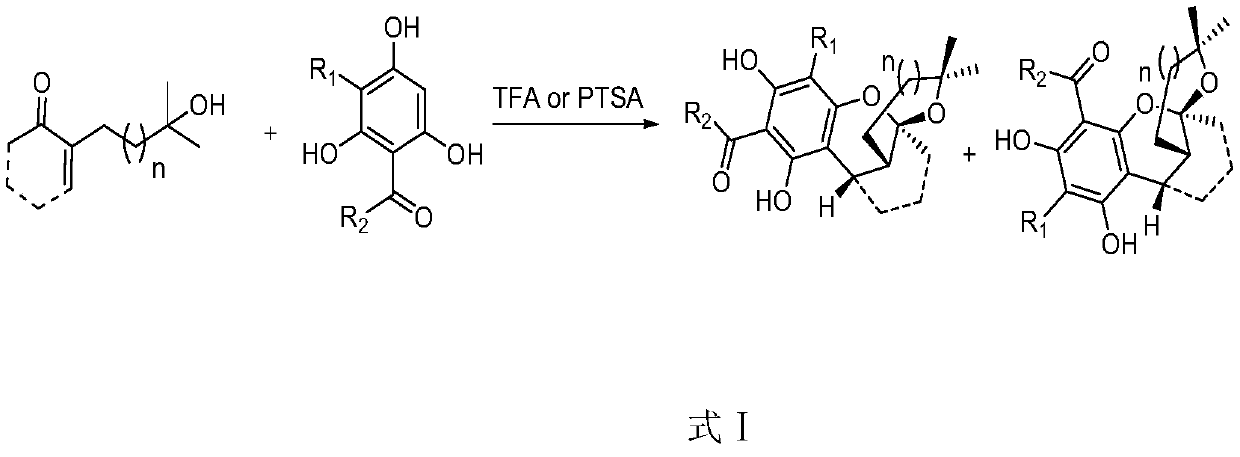
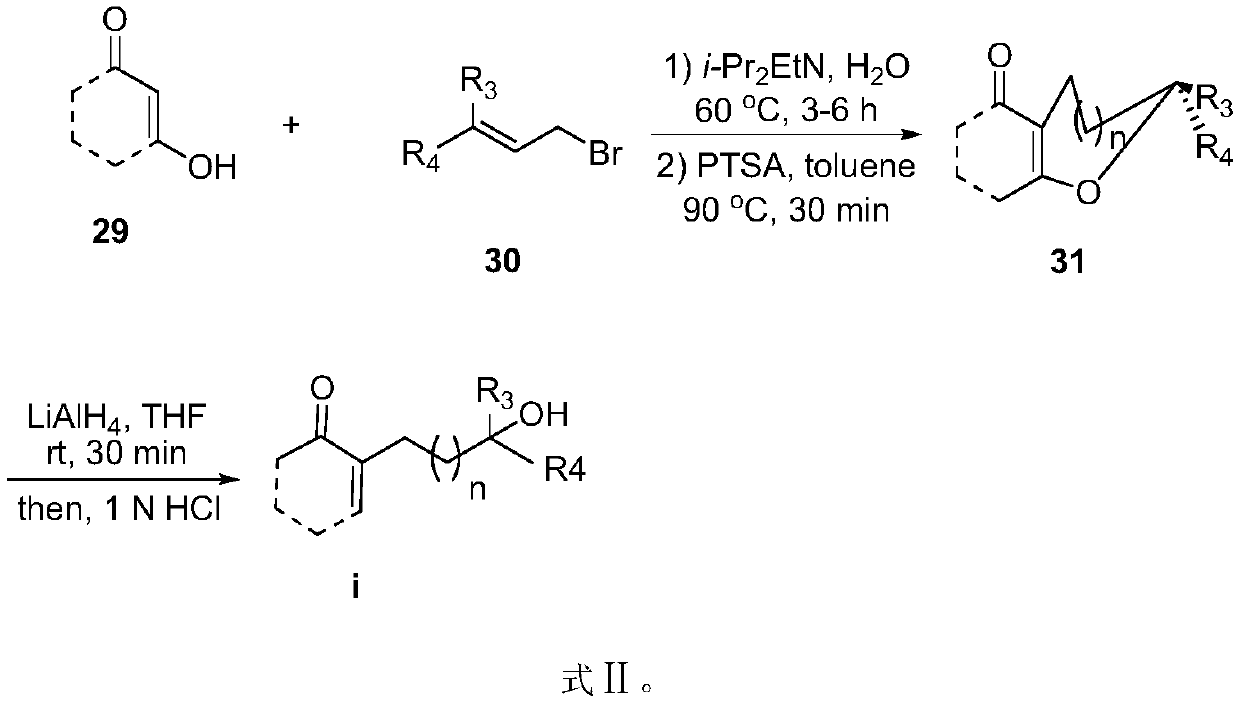
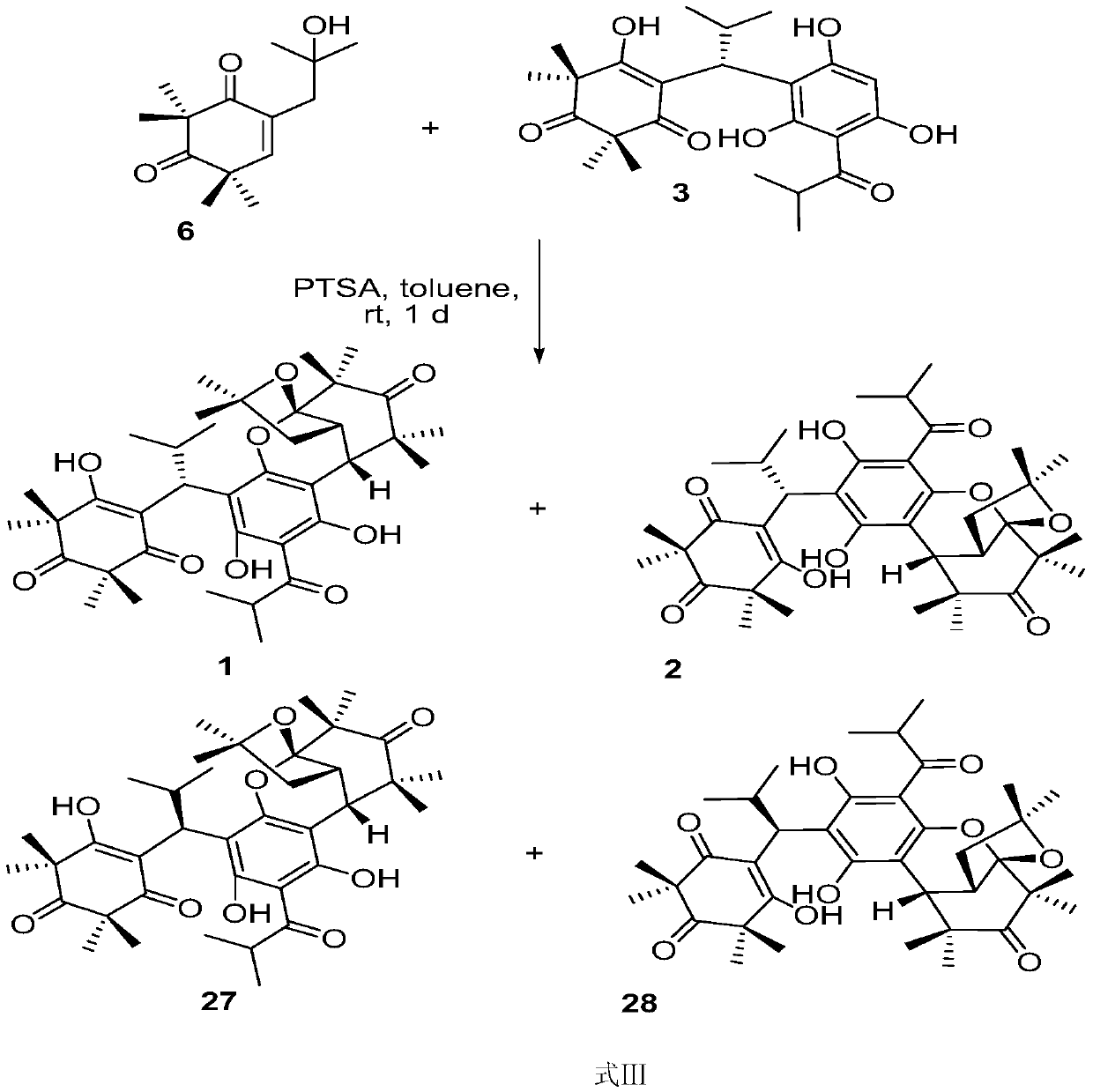
Preparation methods of myrtuco mmulone J and Myrtucommuacetalone and their analogs
A technology of analogs and ketones, applied in the field of synthetic medicinal chemistry, can solve the problems of no chemical total synthesis, and achieve the effect of economical preparation methods
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0039] Embodiment 1: Screening of reaction conditions
[0040] Taking the reaction of unsaturated ketone (compound 9, 0.2mmol) and isobutyrylphloroglucinol (compound 10, 0.2mmol) in solvent (3mL) as an example, investigate the influence of different solvents, acid catalysts and reaction temperature, the specific steps are as follows :
[0041] Dissolve unsaturated ketone (compound 9, 0.2mmol) and isobutyrylphloroglucinol (compound 10, 0.2mmol) in the solvent, add trifluoroacetic acid (50uL) or p-toluenesulfonic acid (0.02- 0.05mmol) at different temperatures. After the reaction was completed, the mixture was purified through a section of about 10 cm long silica gel chromatography column (n-hexane:ethyl acetate=5:1) to obtain the corresponding products Compounds 11 and 12. Spectral data: 1 H NMR (500MHz, CDCl 3 ):δ=14.03(brs,1H),13.92(brs,1H),6.15(brs,1H),5.93(s,1H),5.83(s,1H),4.00(dt,J=13.4,6.5Hz, 1H), 3.89(dt, J=13.6, 6.8Hz, 1H), 3.52(d, J=2.8Hz, 1H), 3.47(d, J=3.0Hz, 1H...
Embodiment 2
[0050] Example 2: Different unsaturated ketone compounds
[0051] The specific steps of synthesis are as follows:
[0052] Dissolve unsaturated ketone (compound 16, 0.2mmol) and isobutyrylphloroglucinol (compound 10, 0.2mmol) in toluene / tetrahydrofuran mixed solution (3mL, v:v=5:1), and add to the reaction system Add trifluoroacetic acid (50uL) or p-toluenesulfonic acid (0.02-0.05mmol) to react. After the reaction was completed, the mixture was purified through a silica gel chromatography column (n-hexane:ethyl acetate=5:1) about 10 cm long to obtain the corresponding products Compound 17 and Compound 18.
[0053]
[0054] 1-(6,8-Dihydroxy-2,2-dimethyl-3,4,4a,5-tetrahydro-2H-5,10a-propanopyrano[2,3-b]chromen-7-yl)-2-methylpropan- 1-one(compound 17a and 1-(6,8-dihydroxy-2,2-dimethyl-3,4,4a,5-tetrahydro-2H-5,10a-propanopyrano[2,3-b]chromen-9- yl)-2-methylpropan-1-one (compound 18a):
[0055]
[0056] 1 H NMR (500MHz, CDCl 3 ):δ=13.84(brs,1H),13.70(brs,1H),6.33(brs,1H...
Embodiment 3
[0076] Example 3: Different monoacylphloroglucinol compounds
[0077] When different monoacylphloroglucinol compounds 13a-13f were used as substrates, the target analogs could be efficiently obtained in very good yields under the conditions of trifluoroacetic acid as catalyst and toluene / THF as mixed solvent .
[0078] Two, when diformylphloroglucinol is used, p-toluenesulfonic acid is needed as a catalyst, toluene is used as a solvent, and the target product can be successfully obtained under the condition of heating at 60 degrees.
[0079]
[0080] Spectral data: 1-(5,7-Dihydroxy-2,2-dimethyl-2,3,3a,4-tetrahydro-4,9a-propanofuro[2,3-b]chromen-6-yl)etha none (compound 14a) 1-(5,7-dihydroxy-2,2-dimethyl-2,3,3a,4-tetrahydro-4,9a-propanofuro[2,3-b]chromen-8-yl)etha none (Compound 15a ):
[0081]
[0082] 1 H NMR (500MHz, CDCl 3 ):δ=13.87(s,1H),5.97(s,1H),5.97(s,1H),3.84(ddd,J=6.6,4.2,2.6Hz,1H),3.53(d,J=2.8Hz, 1H), 3.48(d, J=2.8Hz, 1H), 2.71(s, 3H), 2.70(s, 3H), 2.37-...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com