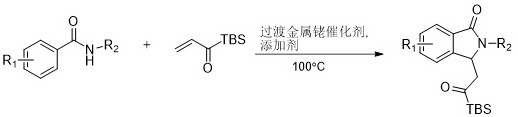
A kind of rhodium-catalyzed carbon-hydrogen activation reaction synthesizes the method for the isoindol-1-one analog of acyl silicon substitution
A technology of hydrocarbon activation and acyl silicon, applied in the field of isoindol-1-one analogs, can solve the problems of limiting the synthesis range of acyl silane, harsh reaction conditions, uneconomical and the like, and achieves the effect of wide substrate applicability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment example 1
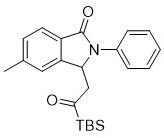
[0020] Implementation Case 1: Synthesis of Compound 1
[0021]
[0022] Under the condition of argon, add in sequence in a clean pressure-resistant bottle N -Substituted benzamide (21.1mg, 0.1mmol), 1-(tert-butyldimethylsilyl)prop-2-en-1-one (51mg, 0.3mmol), dichloro(pentamethylcyclopentyl Dienyl) rhodium (Ⅲ) (3.1mg, 0.005mmol), silver carbonate (55.2mg, 0.2mmol), acetonitrile (1.5ml), placed in an oil bath at 100°C and stirred for 36h.
[0023] After the reaction, the solvent was removed under reduced pressure, and the product was purified by silica gel column chromatography to obtain the product, namely a light yellow solid with a melting point range of 150.5-151.7° C. and a yield of 83%. 1 H NMR (400 MHz, Chloroform- d ) δ 7.79 (d, J = 7.8Hz, 1H), 7.55 (d, J = 7.4 Hz, 2H), 7.44 – 7.38 (m, 2H), 7.29 (d, J = 7.8 Hz,1H), 7.20 (t, J = 7.4 Hz, 1H), 7.17 (s, 1H), 5.82 (dd, J = 8.4, 3.2 Hz, 1H),3.13 (dd, J = 18.6, 3.2 Hz, 1H), 2.82 (dd, J = 18.6, 8.5 Hz, 1H), 2....
Embodiment example 2
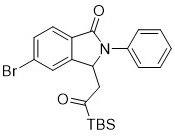
[0024] Implementation Case 2: Synthesis of Compound 2
[0025]
[0026] Under the condition of argon, add in sequence in a clean pressure-proof bottle N -Substituted benzamide (27.5mg, 0.1mmol), 1-(tert-butyldimethylsilyl)prop-2-en-1-one (51mg, 0.3mmol), bis(hexafluoroantimonate)tri Acetonitrile (pentamethylcyclopentadienyl) rhodium (III)) (8.3mg, 0.01mmol), silver carbonate (55.2mg, 0.2mmol), acetonitrile (1.5ml), placed in a 100°C oil bath and stirred for 36h.
[0027] After the reaction, the solvent was removed under reduced pressure, and the product was purified by silica gel column chromatography to obtain the product, that is, a light yellow solid with a melting point range of 169.5-171.3° C. and a yield of 75%. 1 H NMR (400 MHz, Chloroform- d ) δ 7.77 (d, J = 8.1Hz, 1H), 7.63 (dd, J = 8.1, 1.7 Hz, 1H), 7.56 (s, 1H), 7.52 (dd, J = 8.7, 1.2Hz, 2H), 7.45 – 7.40 (m, 2H), 7.26 – 7.20 (m, 1H), 5.81 (dd, J = 8.6, 3.1 Hz,1H), 3.16 (dd, J = 18.7, 3.2 Hz, 1H), 2.81...
Embodiment example 3
[0028] Implementation Case 3: Synthesis of Compound 3
[0029]
[0030] Under the condition of argon, add in sequence in a clean pressure-resistant bottle N-Substituted benzamide (22.7mg, 0.1mmol), 1-(tert-butyldimethylsilyl)prop-2-en-1-one (51mg, 0.3mmol), dichloro(pentamethylcyclopentyl Dienyl) rhodium (Ⅲ) (6.2mg, 0.01mmol), silver acetate (33.4mg, 0.2mmol), tetrahydrofuran (1.5ml), placed in an oil bath at 100°C and stirred for 36h.
[0031] After the reaction, the solvent was removed under reduced pressure, and the product was purified by silica gel column chromatography to obtain the product, a light yellow solid with a melting point range of 94.9-96.3°C and a yield of 55%. 1 H NMR (400 MHz, Chloroform- d ) δ 7.89 (d, J = 7.0 Hz,1H), 7.54 (td, J = 7.4, 1.4 Hz, 1H), 7.50 – 7.44 (m, 1H), 7.43 – 7.39 (m,2H), 7.37 (d, J = 6.4 Hz, 1H), 6.98 – 6.91 (m, 2H), 5.76 (dd, J = 8.2, 3.6Hz, 1H), 3.82 (s, 3H), 3.12 (dd, J = 18.6, 3.6 Hz, 1H), 2.80 (dd, J = 18.6,8.2 Hz, ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com