Novel five-substituted 2,3-dihydropyrrole derivative and preparation method and application thereof
A technology of dihydropyrrole and derivatives, which is applied in the application of antidiabetic drugs and the field of preparation of 2,3-dihydropyrrole compounds, can solve the problems of not being widely used, demanding reaction conditions, environmental pollution, etc., and achieves High stereoselectivity, good inhibitory activity, and good atom economy
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] Synthesis of compound 4a.
[0031]
[0032] Take 62mg (0.5mmol) p-methoxyaniline, p-tolualdehyde 120mg (1.0mmol) and p-chloropyruvamide 200mg (1.0mmol) in a 100ml round bottom flask, and dissolve it with 20ml EtOH, stir for 5 After 1.6 ml acetic acid was added. The reaction bottle was placed in an 80°C oil bath and heated to reflux for 8 hours. After cooling to room temperature, it was concentrated under reduced pressure to remove ethanol, and the residue was diluted with ethyl acetate and water. After liquid separation, the aqueous phase was extracted two more times with ethyl acetate. The combined organic phases were successively washed with saturated KHSO 4 Aqueous solution, saturated NaHCO 3 Washed with aqueous solution and saturated brine, anhydrous MgSO 4 Dry and concentrate in vacuo to give crude product. PE / EA (5:1~3:1) was purified by silica gel column chromatography to obtain 178 mg of product 4a with a yield of 51%. Structural parameters: 1 H NMR (...
Embodiment 2
[0034] Synthesis of compound 4b.
[0035]
[0036] The synthetic method of embodiment 2 is the same as above-mentioned synthetic general method.
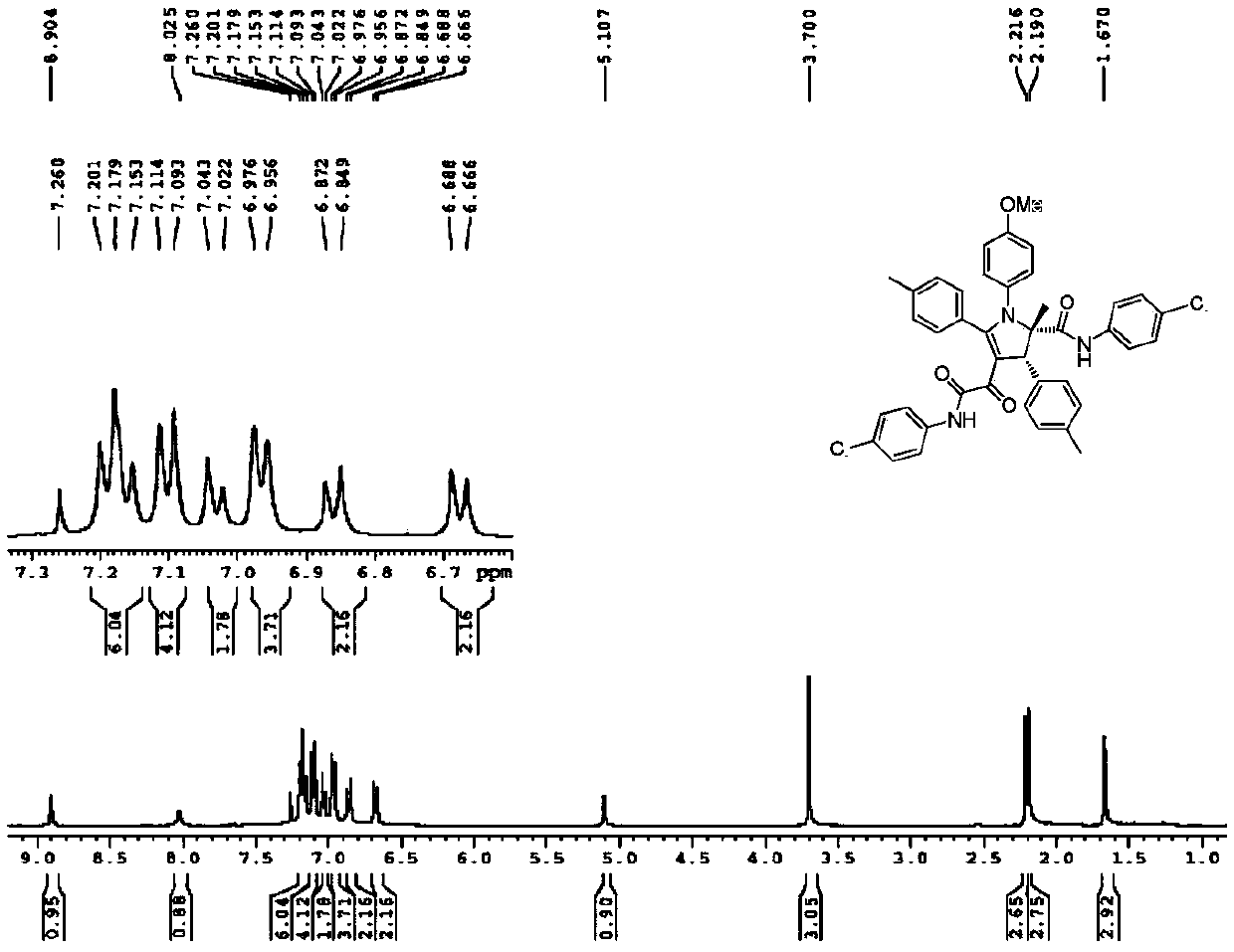
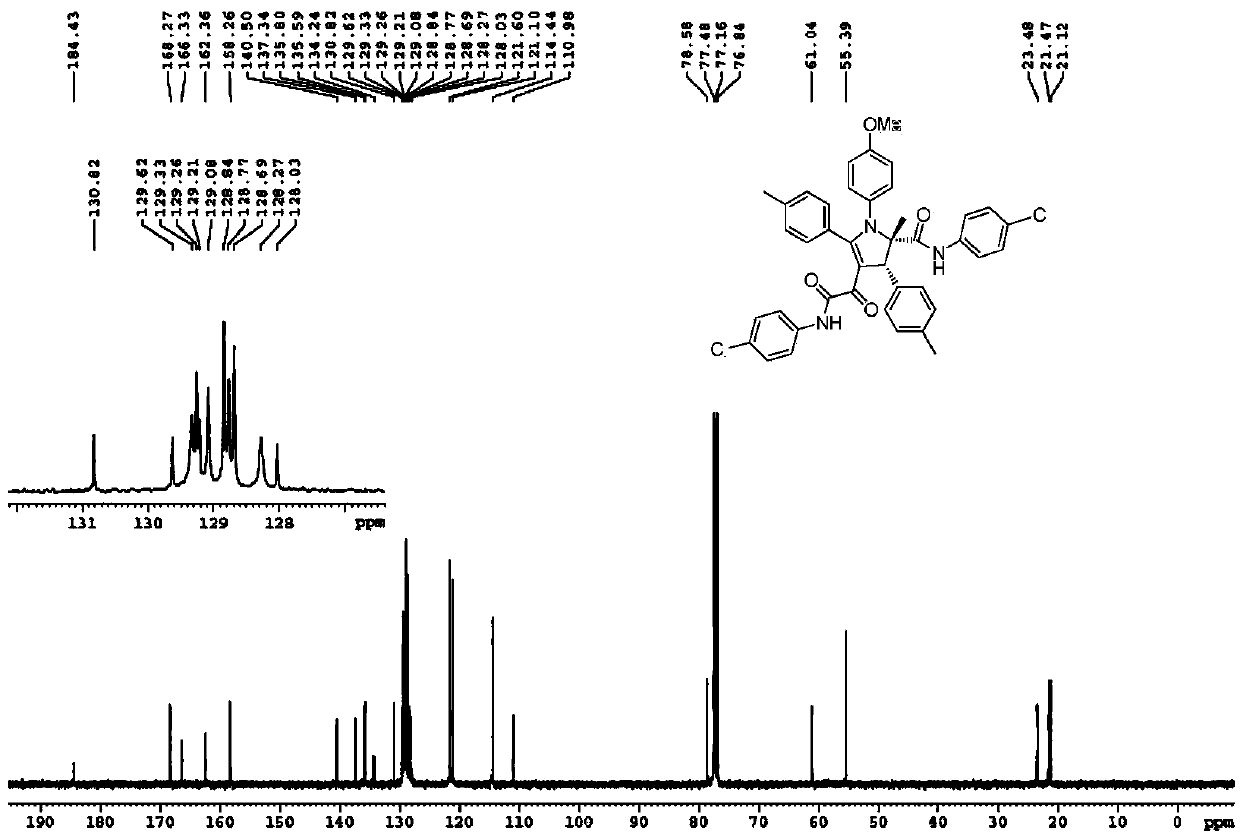
[0037] Yield: 49%; Structural parameters: 1 H NMR (400MHz, CDCl 3 )δ8.63(s,1H),7.79(s,1H),7.22-7.20(m,3H),7.18-7.13(m,4H),7.11-7.09(m,1H),7.05-6.95(m, 8H), 6.88(d, J=8.8Hz, 2H), 6.68(d, J=8.8Hz, 2H), 5.16(s, 1H), 3.71(s, 3H), 2.28(s, 3H), 2.20( s,3H),1.70(s,3H). 13 C NMR (100MHz, CDCl 3 )δ184.2,168.4,166.5,162.6,162.5,158.3,140.6,138.3,138.1,137.5,134.4,134.3,130.9,129.73,129.67,129.4,129.3,129.1,128.9,128.3,128.0,124.8,124.3,120.8,120.1 ,118.7,118.0,114.4,111.0,78.7,60.8,55.4,23.7,21.4,21.1.HRMS(ESI-TOF)m / z calcd.for C 41 h 36 N 3 o 4 Cl 2 [M+H] + :704.2077,found704.2076.
Embodiment 3
[0039] Synthesis of compound 4c.
[0040]
[0041] The synthetic method of embodiment 3 is the same as above-mentioned synthetic general method.
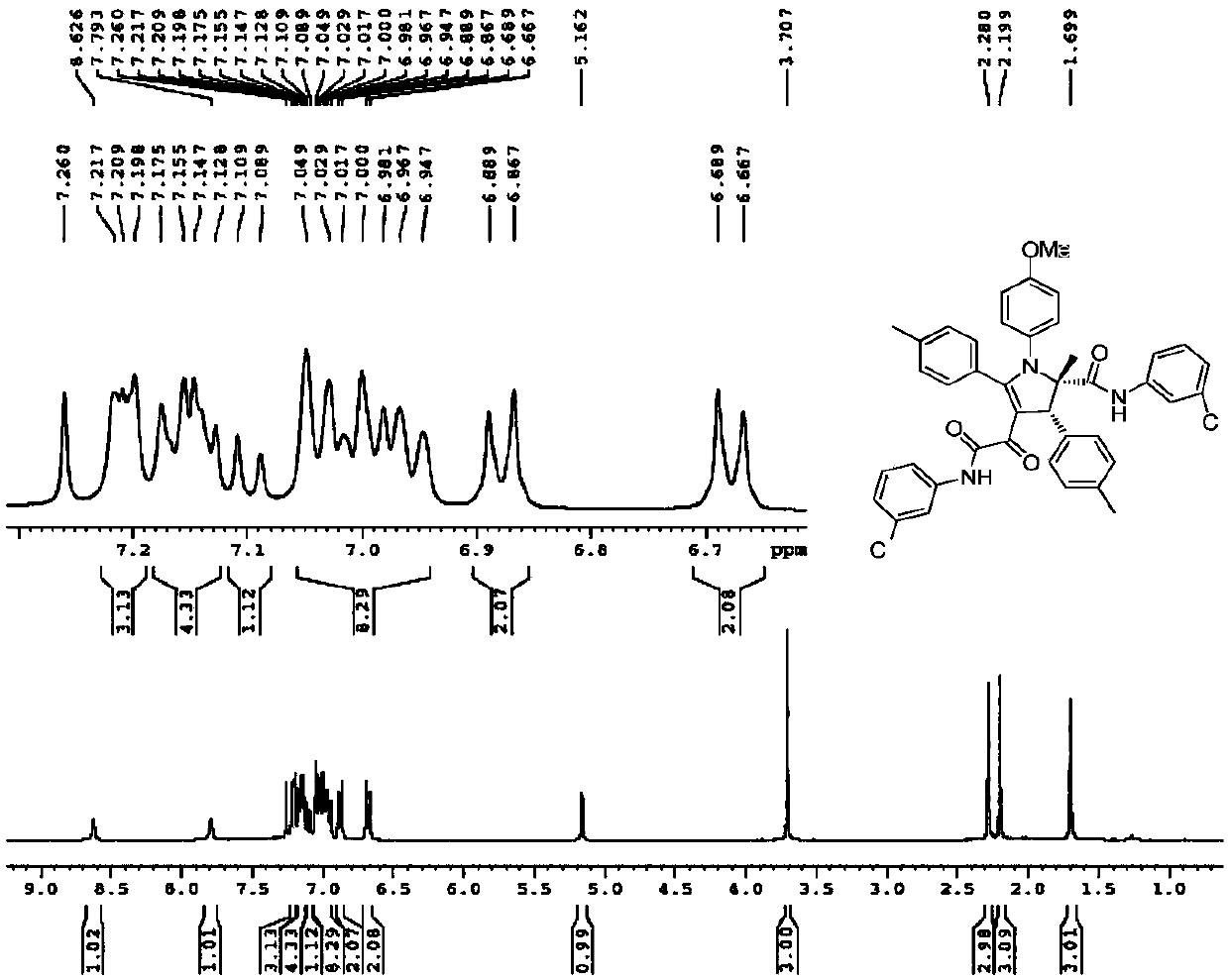
[0042] Yield: 62%; Structural parameters: 1 H NMR (400MHz, CDCl 3)δ8.93(s,1H),8.79(s,1H),7.70(dd,J=8.0,14.0Hz,2H),7.37-7.35(m,1H),7.32-7.29(m,2H),7.271 -7.269(m,1H),7.18(d,J=8.0Hz,2H),7.14-7.06(m,4H),7.02-6.97(m,1H),6.94(d,J=8.8Hz,3H), 6.90(d,J=7.6Hz,2H),6.66(d,J=8.8Hz,2H),5.28(s,1H),3.70(s,3H),2.28(s,3H),2.10(s,3H ),1.65(s,3H).HRMS(ESI-TOF)m / z calcd.for C 41 h 36 N 3 o 4 Cl 2 [M+H] + :704.2077,found704.2079.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com