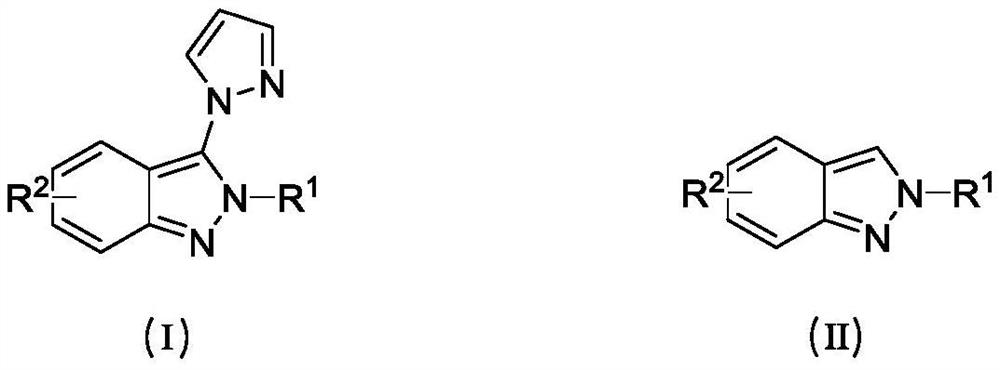Method for green oxidation synthesis of N-substituted 2H-indazole compound
A technology for oxidative synthesis and compound, applied in the direction of organic chemistry, can solve problems such as limited application prospects, and achieve the effects of high product yield, wide substrate applicability, and easy operation.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0020]
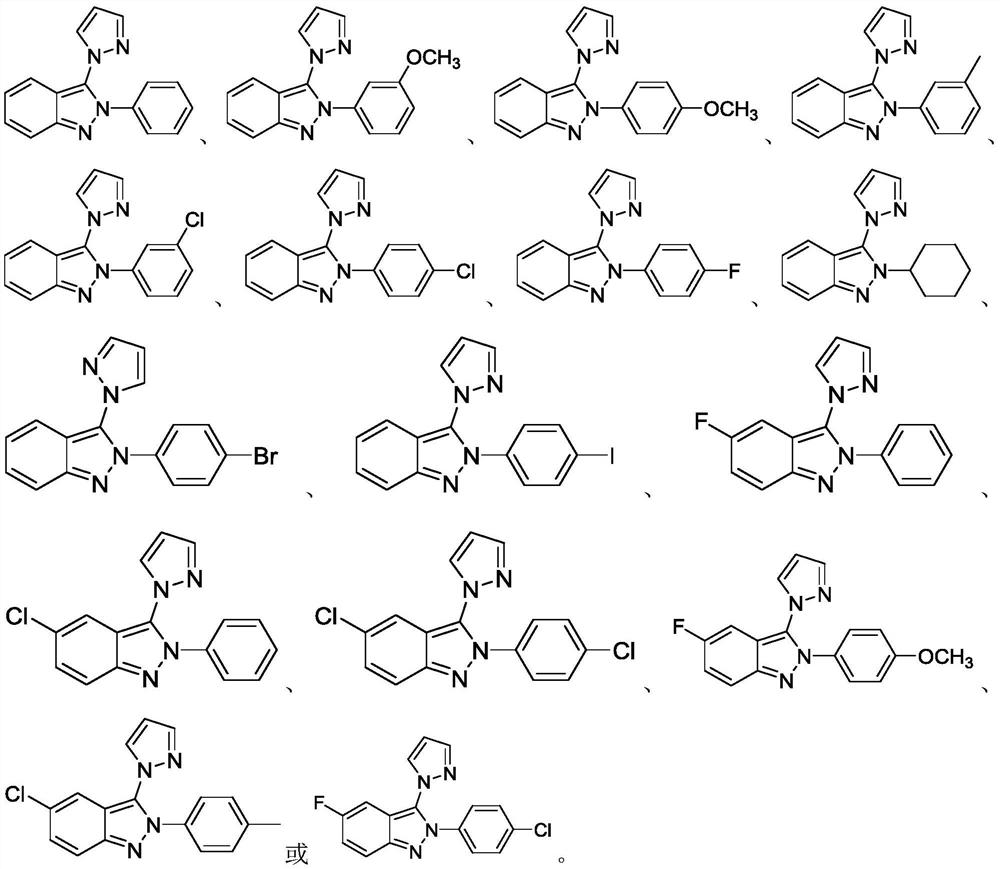
[0021] 2-Phenyl-2H-indazole (0.3mmol, 58.2mg), pyrazole (1.5eq., 30.6mg), potassium persulfate (3.0eq., 243mg), and water 3mL were sequentially added into a 25mL Schleck tube. Reaction at 80°C for 12h. After the reaction was completed (monitored by TLC), the heating was stopped, and after cooling to room temperature, ethyl acetate (EA) was added for extraction (3×10 mL), the organic layers were combined and dried, concentrated under reduced pressure, column chromatography, and petroleum ether: ethyl acetate The 80:1 ester mixture was used as the eluent, and the eluent containing the target product was collected and distilled under reduced pressure to obtain 70.2 mg of the target product as a white solid, with a yield of 90%.
[0022] M.p.132-133℃. 1 H NMR (400MHz, CDCl 3 )δ7.85(d, J=1.8Hz, 1H), 7.81(d, J=8.8Hz, 1H), 7.60(d, J=8.6Hz, 1H), 7.55(d, J=2.4Hz, 1H) ,7.44–7.38(m,6H),7.21(dd,J=8.2,6.8Hz, 1H),6.50(t,J=2.0Hz,1H). 13 C NMR (100MHz, CDCl 3 )δ148.02,142.46,...
Embodiment 2
[0024]
[0025] In a 25mL Schleck tube, add 2-(3-methoxyphenyl)-2H-indazole (0.3mmol, 67.2mg), pyrazole (1.5 eq., 30.6mg), potassium persulfate (4.0eq., 324mg ), water 3mL. Reaction at 60°C for 12h. After the reaction was completed (monitored by TLC), the heating was stopped, and after cooling to room temperature, ethyl acetate (EA) was added for extraction (3×10 mL), the organic layers were combined and dried, concentrated under reduced pressure, column chromatography, and petroleum ether: ethyl acetate The 80:1 ester mixture was used as the eluent. The eluent containing the target product was collected and distilled under reduced pressure to obtain 72.2 mg of the target product as a white solid with a yield of 83%.
[0026] M.p.93-94℃. 1 H NMR (400MHz, CDCl 3 )δ7.87(d, J=1.8Hz, 1H), 7.81(d, J=8.8Hz, 1H), 7.59(d, J=8.6Hz, 1H), 7.56(d, J=2.4Hz, 1H) ,7.41(dd,J=8.2,7.2Hz,1H),7.31(d,J=7.8Hz, 1H),7.21(dd,J=8.4,6.8Hz,1H),6.98–6.93(m,3H), 6.51(t,J=2.0Hz,1H),3.77(s,3H). 13 C...
Embodiment 3
[0028]
[0029] Add 2-(4-methoxyphenyl)-2H-indazole (0.3mmol, 67.2mg), pyrazole (1.5eq., 30.6mg), potassium persulfate (2.0eq., 163mg), water 3mL. Reaction at 80°C for 12h. After the reaction was completed (monitored by TLC), the heating was stopped, and after cooling to room temperature, ethyl acetate (EA) was added for extraction (3×10 mL), the organic layers were combined and dried, concentrated under reduced pressure, column chromatography, and petroleum ether: ethyl acetate The 80:1 ester mixture was used as the eluent, and the eluent containing the target product was collected and distilled under reduced pressure to obtain 56.5 mg of the target product as a white solid, with a yield of 65%.
[0030] M.p.100-101℃. 1 H NMR (400MHz, CDCl 3 )δ7.84(d, J=1.6Hz, 1H), 7.79(d, J=8.8Hz, 1H), 7.59(d, J=8.6Hz, 1H), 7.53(d, J=2.4Hz, 1H) ,7.41–7.36(m,1H),7.35–7.31(m,2H),7.19 (dd,J=8.2,7.0Hz,1H),6.48(t,J=2.0Hz,1H),3.84(s,3H ). 13 C NMR (100MHz, CDCl 3)δ 159.79, 147.82, 142.30...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com