Poly-substituted alkynyl amidine compounds, preparation method and application thereof
A compound and multi-substitution technology, applied in the field of multi-substituted alkyne amidine compounds and their preparation, can solve problems such as few studies, and achieve the effects of convenient operation, simple and easy-to-obtain raw materials, and novel and efficient synthesis method.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
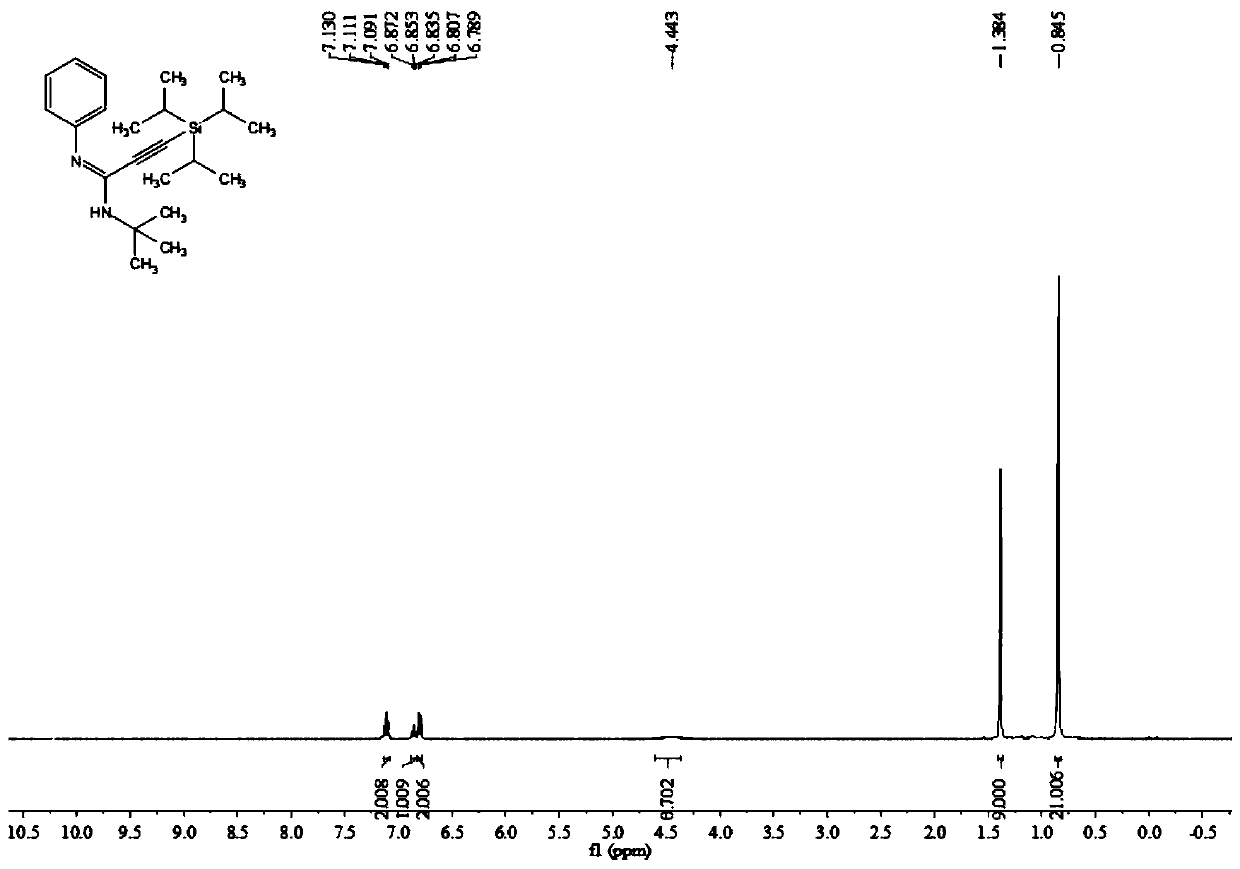
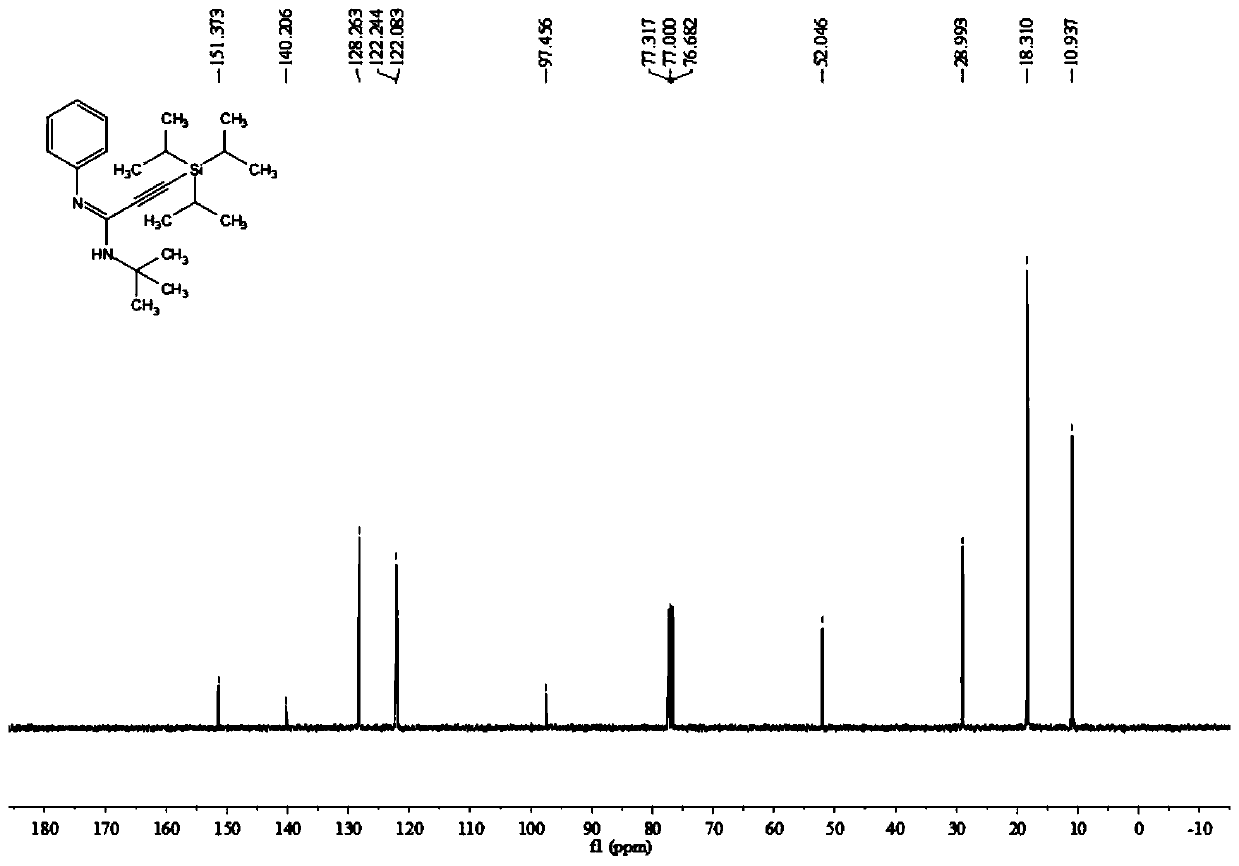
[0046] Add 0.2 mmol of aniline, 0.3 mmol of triisopropylsilyl alkyne bromide, 0.02 mmol of palladium chloride, 0.4 mmol of potassium carbonate, 0.04 mmol of silver trifluoroacetate, and 2 ml of acetonitrile solvent into the reaction tube, and finally Add 0.25 mmol tert-butyl isonitrile, stir and react at 100° C. at 700 rpm for 4 hours, and stop stirring. Add 5 mL of water, extract 3 times with ethyl acetate, combine the organic phases and use 0.5 g of anhydrous sodium sulfate to dry, filter, concentrate under reduced pressure, and then separate and purify by column chromatography to obtain the target product, which is eluted by column chromatography The liquid is petroleum ether:ethyl acetate mixed solvent with a volume ratio of 100:1, and the yield is 85%.
[0047] The hydrogen spectrogram and the carbon spectrogram of the product obtained in this embodiment are respectively as follows figure 1 and figure 2 shown; the structural characterization data are shown below:
[0...
Embodiment 2
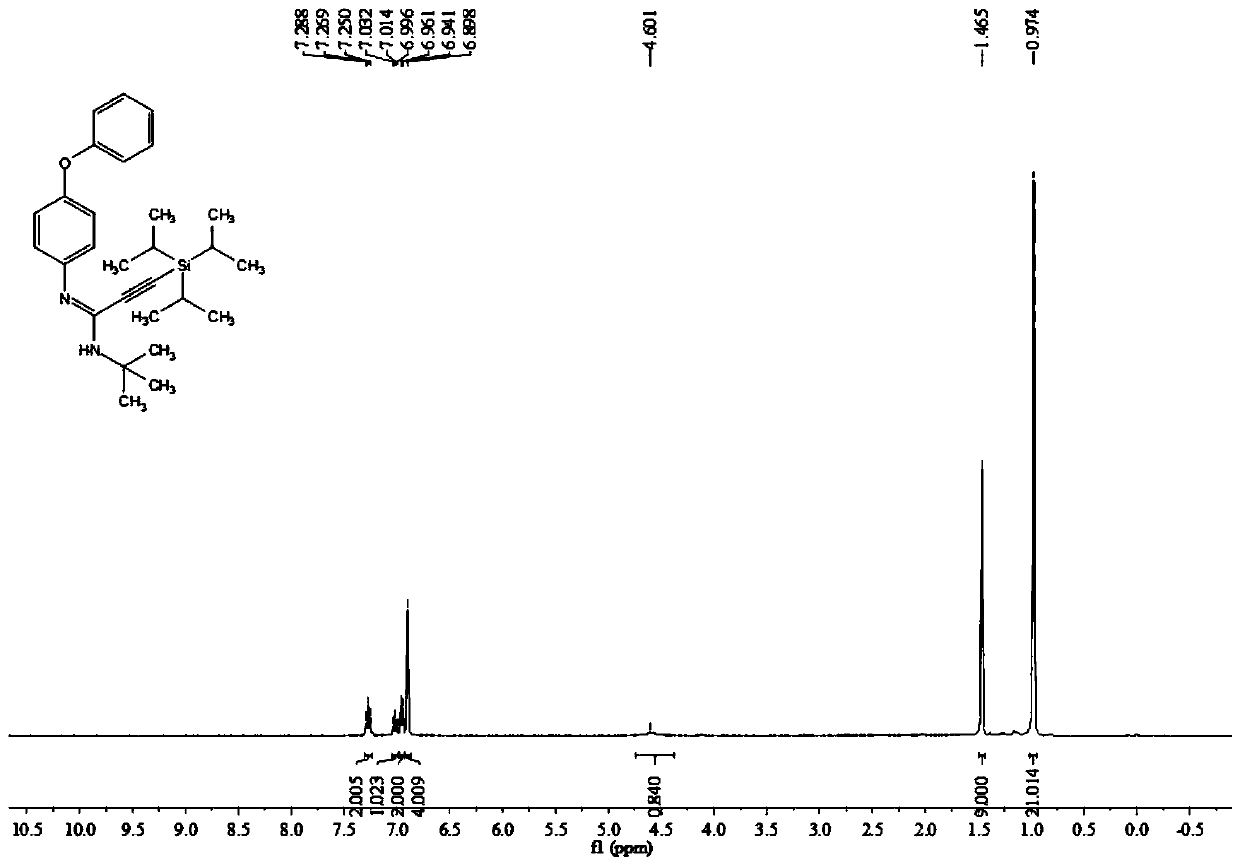
[0055] In the reaction tube, add 0.2 mmol 4-phenoxyaniline, 0.3 mmol triisopropylsilyl alkyne bromide, 0.02 mmol palladium chloride, 0.4 mmol potassium carbonate, 0.04 mmol silver trifluoroacetate, and 2 Milliliter of acetonitrile solvent, finally add 0.25 mmol tert-butyl isonitrile, stir the reaction at 100 degrees Celsius at 700 rpm for 4 hours, stop stirring. Add 5 mL of water, extract 3 times with ethyl acetate, combine the organic phases and use 0.5 g of anhydrous sodium sulfate to dry, filter, concentrate under reduced pressure, and then separate and purify by column chromatography to obtain the target product, which is eluted by column chromatography The liquid is petroleum ether with a volume ratio of 60:1: ethyl acetate mixed solvent, and the yield is 70%.
[0056] The hydrogen spectrogram and the carbon spectrogram of the product obtained in this embodiment are respectively as follows image 3 and Figure 4 shown; the structural characterization data are shown belo...
Embodiment 3
[0064] Add 0.2 mmol 4-iodoaniline, 0.3 mmol triisopropylsilyl alkyne bromide, 0.02 mmol palladium chloride, 0.4 mmol potassium carbonate, 0.04 mmol silver trifluoroacetate, and 2 mL acetonitrile to a reaction tube Solvent, finally add 0.25 mmol tert-butyl isonitrile, stir and react at 100 degrees Celsius at 700 rpm for 4 hours, and stop stirring. Add 5 mL of water, extract 3 times with ethyl acetate, combine the organic phases and use 0.5 g of anhydrous sodium sulfate to dry, filter, concentrate under reduced pressure, and then separate and purify by column chromatography to obtain the target product, which is eluted by column chromatography The liquid is sherwood oil with a volume ratio of 80:1: ethyl acetate mixed solvent, and the yield is 84%.
[0065] The hydrogen spectrogram and the carbon spectrogram of the product obtained in this embodiment are respectively as follows Figure 5 and Figure 6 shown; the structural characterization data are shown below:
[0066] 1 H ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com