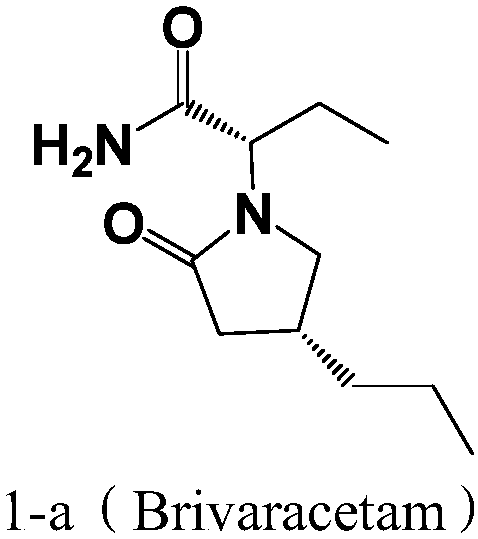
Preparation method of brivaracetam
A technology of temperature control and solvent, which is applied in the field of preparation of buvaracetam, can solve the problems of unfavorable production efficiency, long time-consuming, high production cost, etc., and achieve the advantages of less time-consuming reaction steps, improved production efficiency and production cost Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
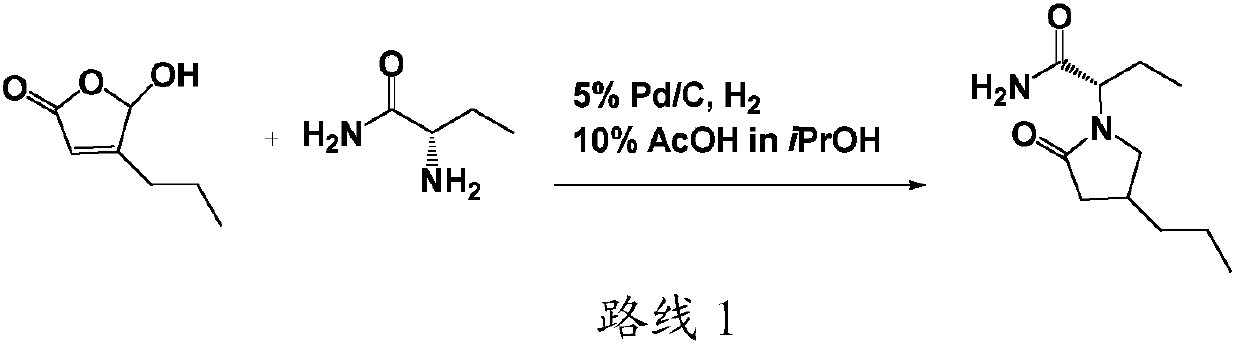
[0077] Hydrogenolysis: Synthesis of Compound 3
[0078]
[0079] In a 1000ml hydrogenation kettle, 450ml of ethyl acetate, 300g (2.11mol) of compound 4, and 10g of 5wt% Pd / C were sequentially added. After the mixture is stirred evenly, seal the hydrogenation tank and use N 2 and H 2 Replace the air in the system. Control the temperature at 25-30°C, H 2 The pressure was controlled at 0.2-0.3MPa, and the mixture system was stirred for 2 hours. Then terminate the reaction, using N in turn 2 and air displacement system H 2 . After the mixture was filtered to separate Pd / C, the solution was distilled off the solvent under reduced pressure at 45-50°C to obtain the crude compound 3 without further treatment, which can be directly put into the subsequent reaction). The reaction time is 2.5 hours.
Embodiment 2
[0081] Hydrogenolysis: Synthesis of Compound 3
[0082]
[0083] In a 1000 ml hydrogenation kettle, 400 ml of isopropyl acetate, 240 g (1.68 mol) of compound 4, and 8 g of 5% Pd / C were successively added. After the mixture is stirred evenly, seal the hydrogenation tank and use N 2 and H 2 Replace the air in the system. Control the temperature at 20-25°C, H 2 The pressure is controlled at 0.15-0.25 MPa, and the mixture system is reacted for 2 hours under stirring condition. Then terminate the reaction, using N in turn 2 and air replacement system H 2 . After the mixture was filtered to separate Pd / C, the solution was distilled off under reduced pressure at 55-60°C to remove the solvent to obtain a crude compound 3 (which can be directly put into subsequent reactions without further treatment). The reaction time was 2.5 hours.
Embodiment 3
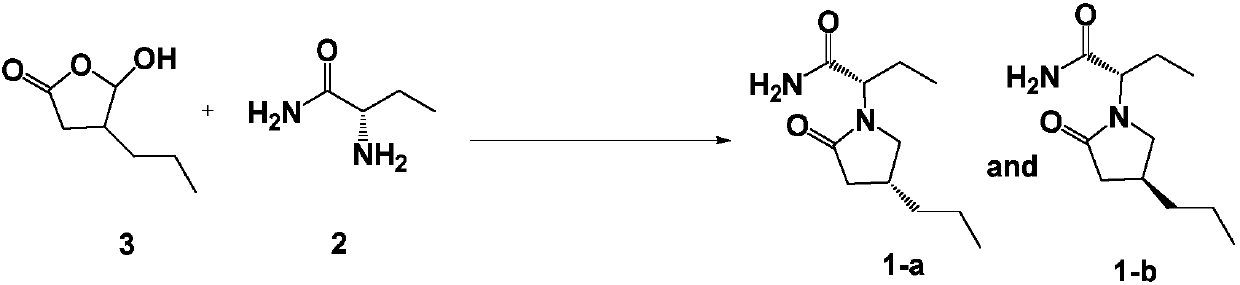
[0085] Reductive amination / lactamization: Synthesis of compound 1 (including 1-a and 1-b)
[0086]
[0087] (1) Amination: In a 1000ml three-necked round-bottomed flask, under a nitrogen atmosphere, 38.8g of compound 2 (0.38mol) was dissolved in 500ml of toluene, the temperature was controlled to 15-20°C, and 49.8g of compound 3 ( 0.34mol) in toluene (150ml) solution. After the dropwise addition was complete, the stirring reaction was continued at this temperature for 3.5 hours.
[0088] (2) Reduction: After the precipitate is completely precipitated, add 52ml of 4mol / L NaOH aqueous solution dropwise to the suspension, then add 8.5g (0.22mol) of sodium borohydride (85ml) solution dropwise, and after about 2.5 hours, add 42ml dropwise The reaction solution was carefully quenched with acetic acid.
[0089] (3) Lactamization: After heating and reacting at 45-50° C. for 3.5 hours, naturally cool to room temperature.
[0090] (4) Post-processing and purification: Add 27.5ml o...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com