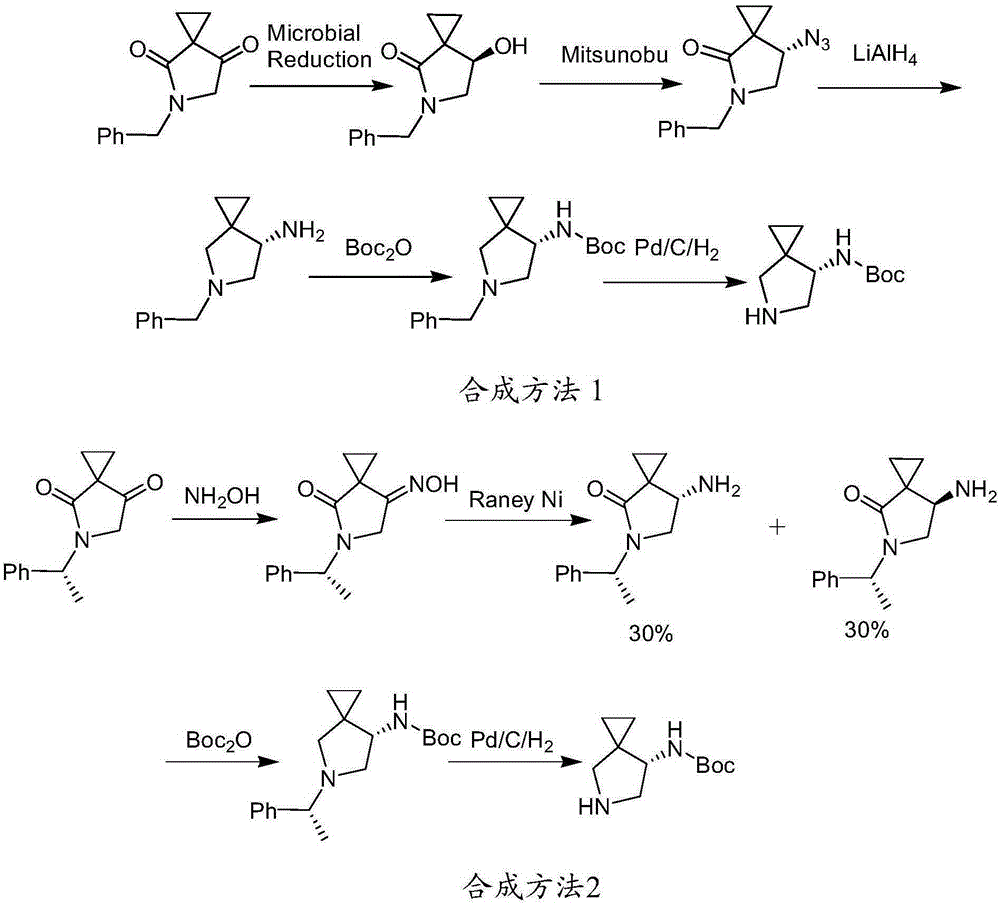
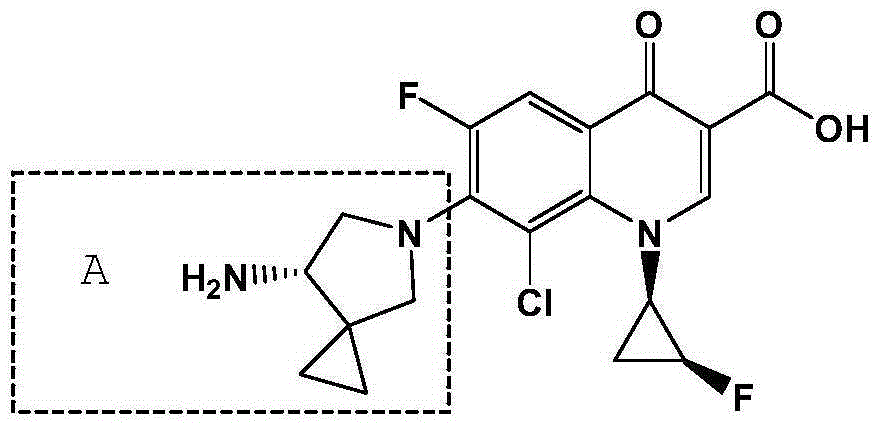
Preparation method of sitafloxacin hydrate five-membered ring side chain intermediate
A sitafloxacin and intermediate technology, which is applied in the field of preparation of sitafloxacin five-membered ring side chain intermediates, can solve the problems of easy explosion of azide compounds, potential safety hazards, high cost, etc. Potential safety hazards and the effects of production costs, low equipment requirements, and mild reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0030] The present invention will be further described below in conjunction with specific embodiments.
[0031] Preparation of starting material 1:
[0032] (1) Bromination of substituted ethyl cyclopropane ketoate
[0033]
[0034] Dissolve 7 g of ethyl substituted cyclopropanone ketoate in 40 ml of absolute ethanol, slowly add 1.2 equivalents of liquid bromine dropwise at 0°C, and react at room temperature for 1 hour after the addition is complete. An aqueous solution of sodium sulfate was used to quench the reaction, and diluted with 60 milliliters of water. After the lower layer product was separated, the aqueous phase was extracted three times with 40 milliliters of ethyl acetate, and the combined extracts were washed once with 40 milliliters of saturated brine, and dried with anhydrous magnesium sulfate After filtration and concentration, the concentrated solution was separated by column chromatography (mobile phase: petroleum ether: ethyl acetate = 30:1) to obtain 7...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com