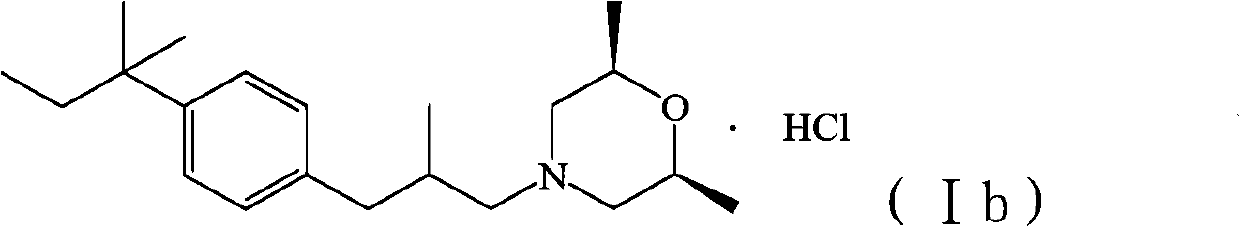
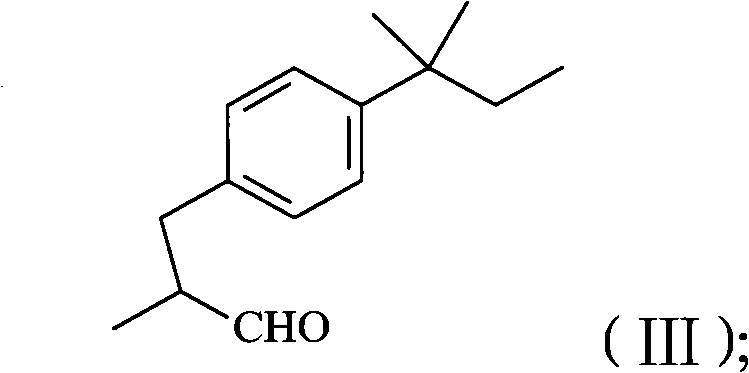
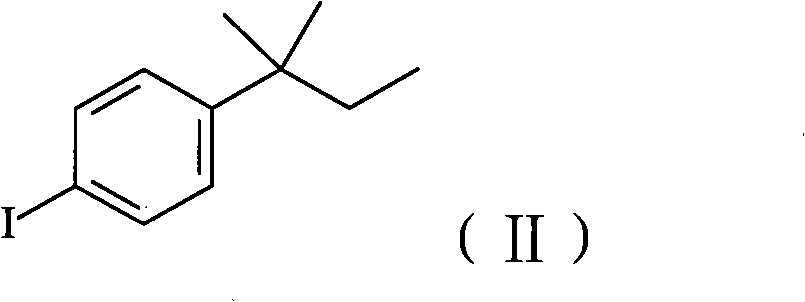Method for preparing amorolfine hydrochloride
A technology of amorolfine hydrochloride and glacial acetic acid, which is applied in the direction of organic chemistry, can solve the problems of not meeting the requirements of high-temperature reaction, unsafe high-pressure reaction equipment, and little application value, and achieves easy control, less side reactions, and overall The effect of high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0058] Add 2600mL of acetic anhydride, 5200mL of glacial acetic acid, 350g of sodium periodate, and 1236g of iodine into a 10L clean reaction kettle, cool to 5°C, add 810mL of sulfuric acid dropwise, control the dropwise addition within 1 hour, and then add 1130g of tert-amylbenzene , stirred at room temperature for more than 16 hours, and the reaction of raw materials was detected by thin-layer chromatography. Pour the reaction solution into 8L of water and 4L of dichloromethane mixture, extract and separate layers, wash the organic layer once with 4L of 25% aqueous sodium sulfite solution, dry the organic layer with anhydrous sodium sulfate, distill off the solvent dichloromethane to obtain a residue It is 2013 g of 4-iodo-tert-amylbenzene, yield: 96%, GC purity 94.2%. 1H-NMR spectrum data: (400MHz, CDCl 3 ): 0.73 (3H, t, J = 7.4Hz), 1.31 (6H, s), 1.67 (2H, q, J = 7.4Hz), 7.13 (2H, d, J = 8.56Hz), 7.66 (2H, d , J=8.56Hz).
Embodiment 2
[0060] In a 10L clean reactor, add 2kg of 4-iodo-tert-amylbenzene prepared according to the method of Example 1, 6L of N-methylpyrrolidone, nitrogen protection, start stirring, add palladium acetate 300g, sodium bicarbonate 1.7kg, Finally, 2.5 kg of 2-methallyl alcohol was added, and the temperature was raised to 105° C. to react, and the GC content of 4-iodo-tert-amylbenzene was detected to monitor the reaction progress, and the reaction was completed in 2 hours. Cool to room temperature, filter, concentrate the filtrate, add 12L of ethyl acetate to dissolve the residue, wash with 20L of water, rectify the organic phase, collect fractions at 125-128°C (vacuum degree ≤ -0.099Mpa), and obtain 3-tert-amylbenzene 1.41 kg of 2-methylpropanal, yield: 88.6%, GC purity: 93.5%. 1H-NMR spectrum data: (400MHz, CDCl 3 ): 0.69 (3H, t, J = 7.45Hz), 1.11 (3H, d, J = 6.87Hz), 1.29 (6H, s), 1.65 (2H, q, J = 7.43Hz), 2.60 (1H, dd , J = 13.52Hz), 2.69 (1H, J = 7.06Hz), 3.08 (1H, dd, J = 13.54...
Embodiment 3
[0063] In a 10L clean reactor, add 2kg of 4-iodo-tert-amylbenzene prepared according to the method in Example 1, 6L of N-methylpyrrolidone, protect with nitrogen, start stirring, add 150g of palladium acetate, 2.5kg of dipotassium hydrogen phosphate , and finally add 1.8kg of 2-methallyl alcohol, heat up to 130°C for reaction, detect the GC content of 4-iodo-tert-amylbenzene to control the reaction process, and the reaction ends in 10 hours. Cool to room temperature, filter, concentrate the filtrate, add 12L ethyl acetate to dissolve the residue, wash with 20L water, concentrate the organic phase, recover ethyl acetate, add the residue dropwise to 10L saturated sodium bisulfite solution at room temperature, and precipitate a solid. Stir for 6 hours, filter, wash the filter cake with 5L of ethyl acetate, disperse the solid in 10L of 1 mol / L hydrochloric acid, stir at room temperature for 5 hours, extract the reaction solution with 10L of ethyl acetate, dry over anhydrous magnesi...
PUM
| Property | Measurement | Unit |
|---|---|---|
| boiling point | aaaaa | aaaaa |
| purity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com