Patents
Literature
48 results about "Gentamicin C1a" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
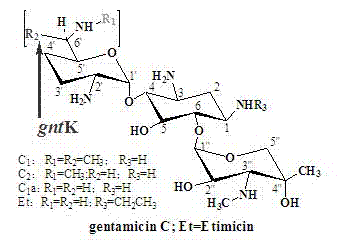
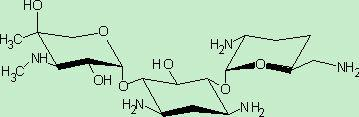
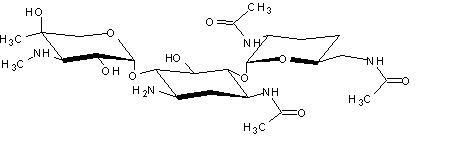
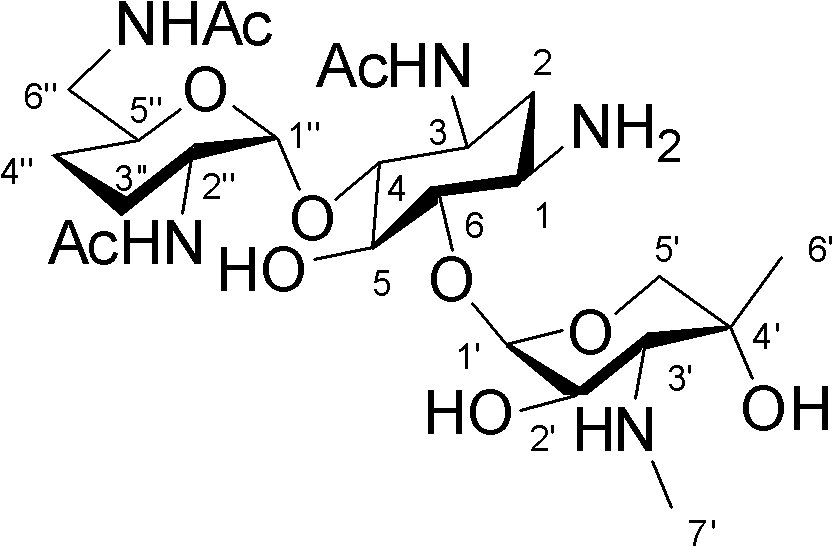
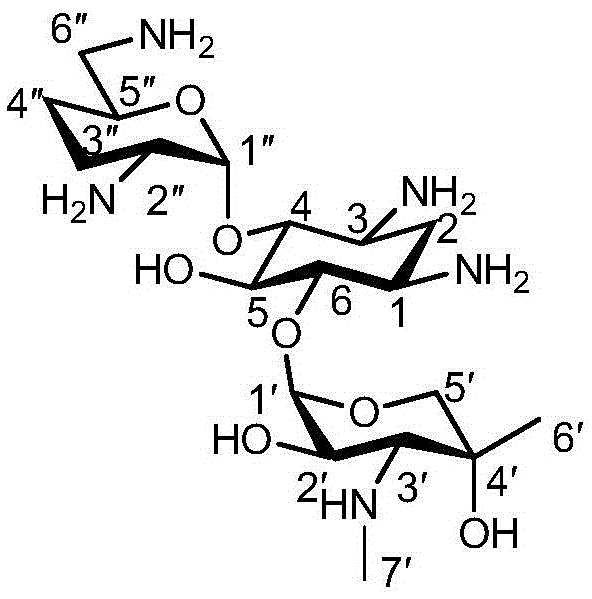
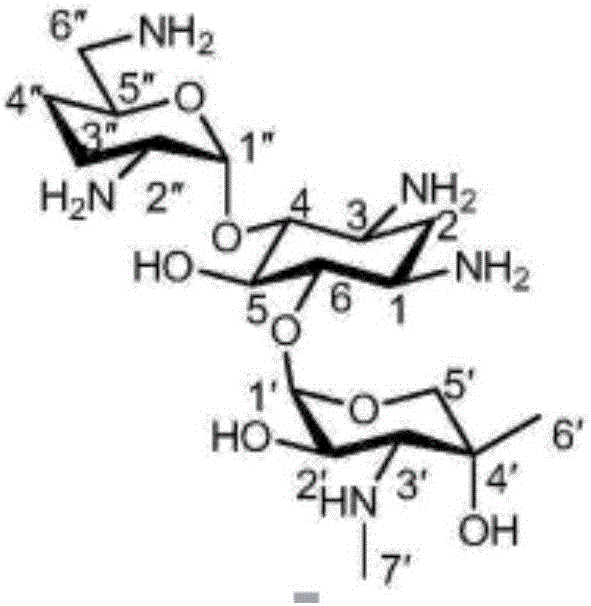
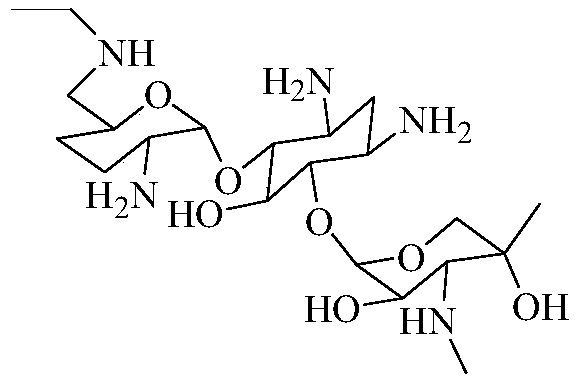
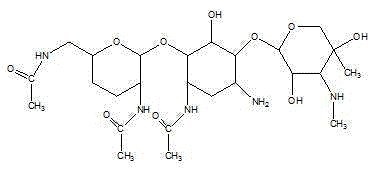
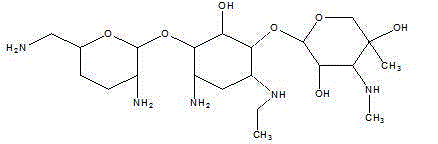
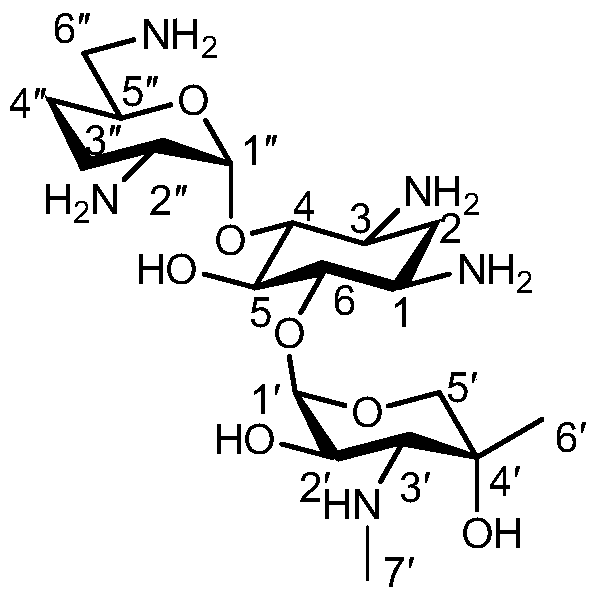
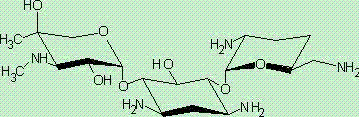
One of the major components of the gentamicin complex. Gentamicin C1a lacks methyl groups on the 2-amino-hexose ring and has a free amine at the 6' position.
Engineering bacteria for producing gentamicin C1a and application thereof
ActiveCN102363759AReduce pollutionSimple production processBacteriaMicroorganism based processesBiotechnologyMicromonospora sp.
The invention belongs to the technical field of medicines and relates to engineering bacteria which are used for producing gentamicin C1a and have a function of deactivating gntK and application of the engineering bacteria. The engineering bacteria are micromonospora purpurea GK1101 which were registered and collected in China General Microbiological Culture Collection Center (CGMCC) in September 13, 2011 and have the collection number of CGMCC No.5245. The engineering bacteria are applied to preparation of antibacterial medicines. The engineering bacteria for producing the gentamicin C1a are obtained; the production process is simplified; production cost is reduced; and quality control over the products is facilitated.
Owner:FUZHOU UNIV +1
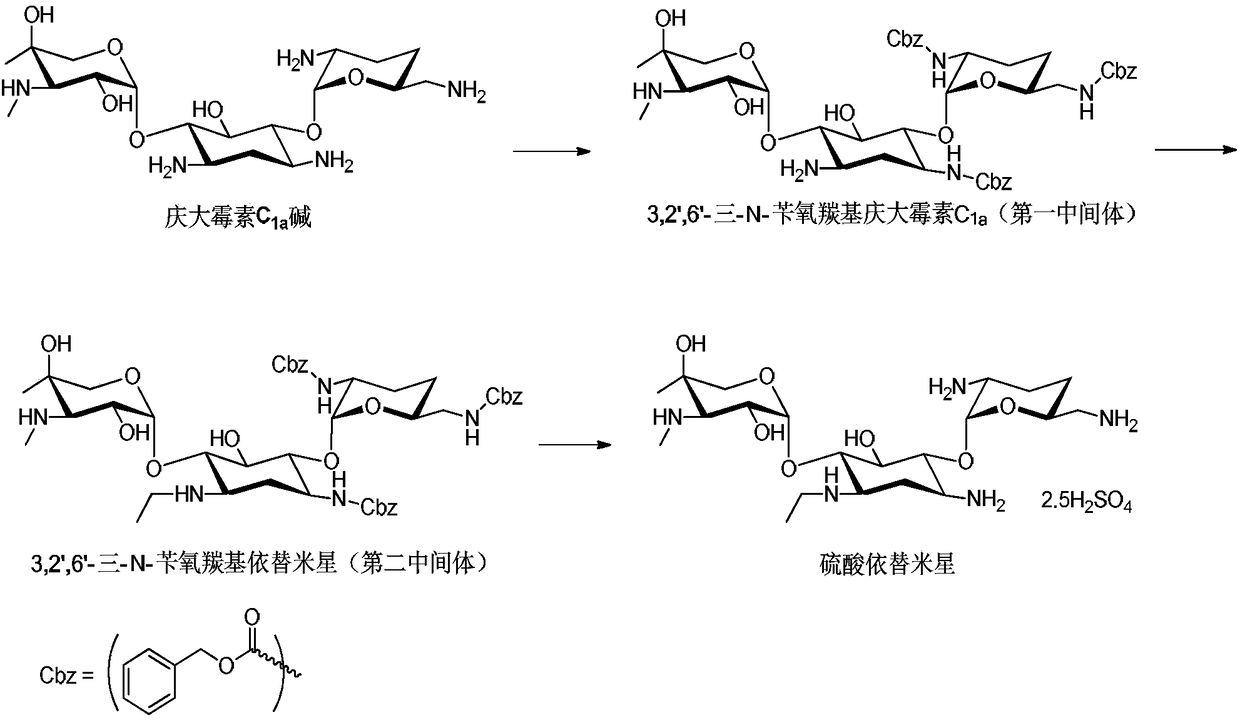
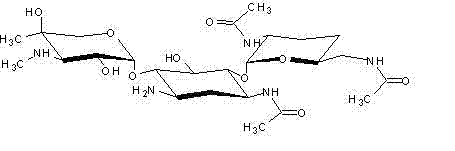
Synthesis method of 3,2',6'-tri-N-acetyl gentamicin Cla
ActiveCN101928309AReduce repulsionHigh yieldSugar derivativesSugar derivatives preparationChromatographic separationAcetic anhydride
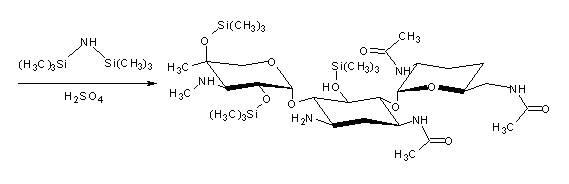
The invention discloses a synthesis method of 3,2',6'-tri-N-acetyl gentamicin Cla, comprising the following steps of: carrying out coordination reaction on gentamicin Cla and zinc acetate in a methanol solent at 15-25 DEG C to form a gentamicin Cla-Zn coordination compound; cooling to 0-10 DEG C, dropwise adding and stirring a mixed solution prepared from acetic anhydride, triethylamine and tetrahydrofuran to the gentamicin Cla-Zn coordination compound, continuing stirring to make the mixture react for 1-2h after finishing dropwise adding the mixed solution, adding water, and carrying out reduced pressure distillation to obtain a concentrated solution containing a 3,2',6'-tri-N-acetyl gentamicin Cla-Zn coordination compound; and introducing the concentrated solution into a chromatographic separation column for sampling, washing with purified water, resolving with an ethanol water, collecting effective constituents at the outlet of the chromatographic separation column, carrying out reduced pressure concentration to a collected solution, and finally freezing and drying to obtain a finished product of the 3,2',6'-tri-N-acetyl gentamicin Cla. The method of the invention has higher yield.
Owner:CHANGZHOU FANGYUAN PHARMA +1
Preparation method of 1-N-ethyl gentamicin C1a sulfate
ActiveCN101928312AReduce repulsionHigh yieldSugar derivativesSugar derivatives preparationWater methanolPotassium borohydride
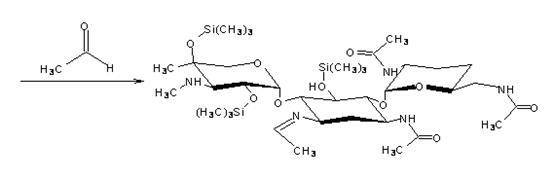
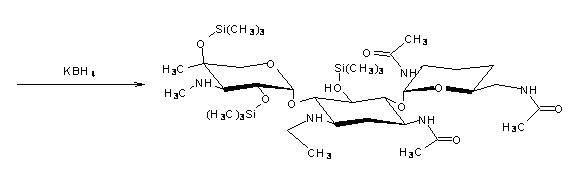
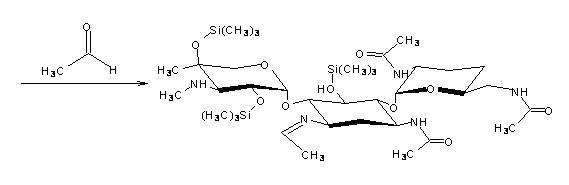
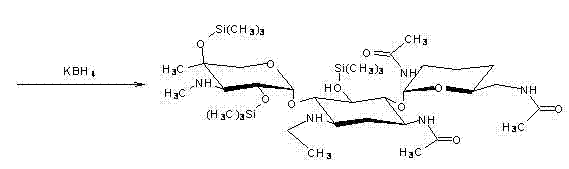
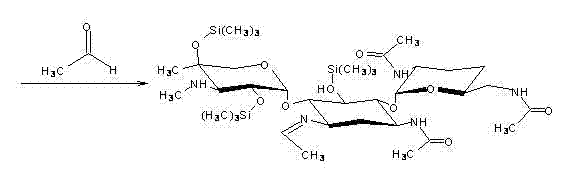
The invention discloses a preparation method of 1-N-ethyl gentamicin C1a sulfate. The method comprises the following steps of: carrying out a complexation reaction on gentamicin C1a and zinc acetate in a methanol solvent; then, dropping a mixed solution of acetic anhydride, triethylamine and tetrahydrofuran to carry out an acylation reaction and obtaining 3,2',6'-3-N-acetyl-gentamicin C1a by post-treatment; then, carrying out a silylation reaction with hexamethyl disilazane in a chloroform solvent; carrying out an N-alkylation reaction with acetaldehyde in a carrene solvent; then, carrying out a reduction reaction with potassium borohydride; hydrolyzing with an NaOH solution; obtaining 1-N-ethyl gentamicin C1a by post-treatment; adding the 1-N-ethyl gentamicin C1a to anhydrous ethanol or anhydrous methanol for stirring or dissolving; then, dropping concentrated sulfuric acid; and obtaining 1-N-ethyl gentamicin C1a sulfate by post-treatment. The method of the invention has higher yield.
Owner:CHANGZHOU FANGYUAN PHARMA +1
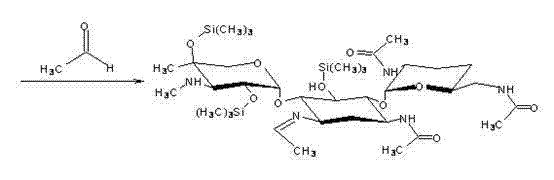
Preparation method of 1-N-ethyl gentamicin Cla
ActiveCN101928311AAvoid Alkylation Side ReactionsAvoid hydrolysisSugar derivativesSugar derivatives preparationAlkyl transferGentamicin C1a
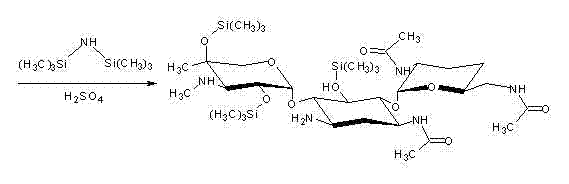
The invention discloses a preparation method of 1-N-ethyl gentamicin Cla, which comprises the following steps of: reacting 3,2',6'-tri-N-acetyl gentamicin Cla with hexamethyldisilazane in a trichloromethane solvent under the catalysis of concentrated sulfuric acid to generate 3,2',6'-tri-N-acetyl-5,2'',4''-tri(trimethylsilyl) gentamicin Cla; carrying out N-alkylation reaction on the 3,2',6'-tri-N-acetyl-5,2'',4''-tri(trimethylsilyl) gentamicin Cla and acetaldehyde in a dichloromethane solvent at a temperature of 8-12 DEG C; carrying out reduction reaction with potassium borohydride; then hydrolyzing with an NaOH solution to obtain hydrolyzate of the 1-N-ethyl gentamicin Cla; and post-processing the hydrolyzate to obtain a finished product of the 1-N-ethyl gentamicin Cla. The method has the advantage of higher yield of the1-N-ethyl gentamicin Cla.
Owner:CHANGZHOU FANGYUAN PHARMA +1
Method for separating and purifying high-purity 3,2'',6''-tri-N-acetyl-gentamicin C1a alkali (P1)
ActiveCN103374047AReduce layoutLess investmentSugar derivativesChemical recyclingChromatographic separationGentamicin C1a
The invention relates to a method for separating and purifying high-purity 3,2'',6''-tri-N-acetyl-gentamicin C1a alkali (P1), belonging to the field of semisynthetic chemical pharmacy. The invention utilizes a continuous chromatographic separation technique in combination with a membrane separation technique to obtain the high-purity 3,2'',6''-tri-N-acetyl-gentamicin C1a alkali (P1) from a P1 cobalt removal solution. The method for separating and purifying P1 has the advantages of high yield, low cost and environmental protection, and is suitable for industrial production.
Owner:WUXI JIMIN KEXIN SHANHE PHARMA +1
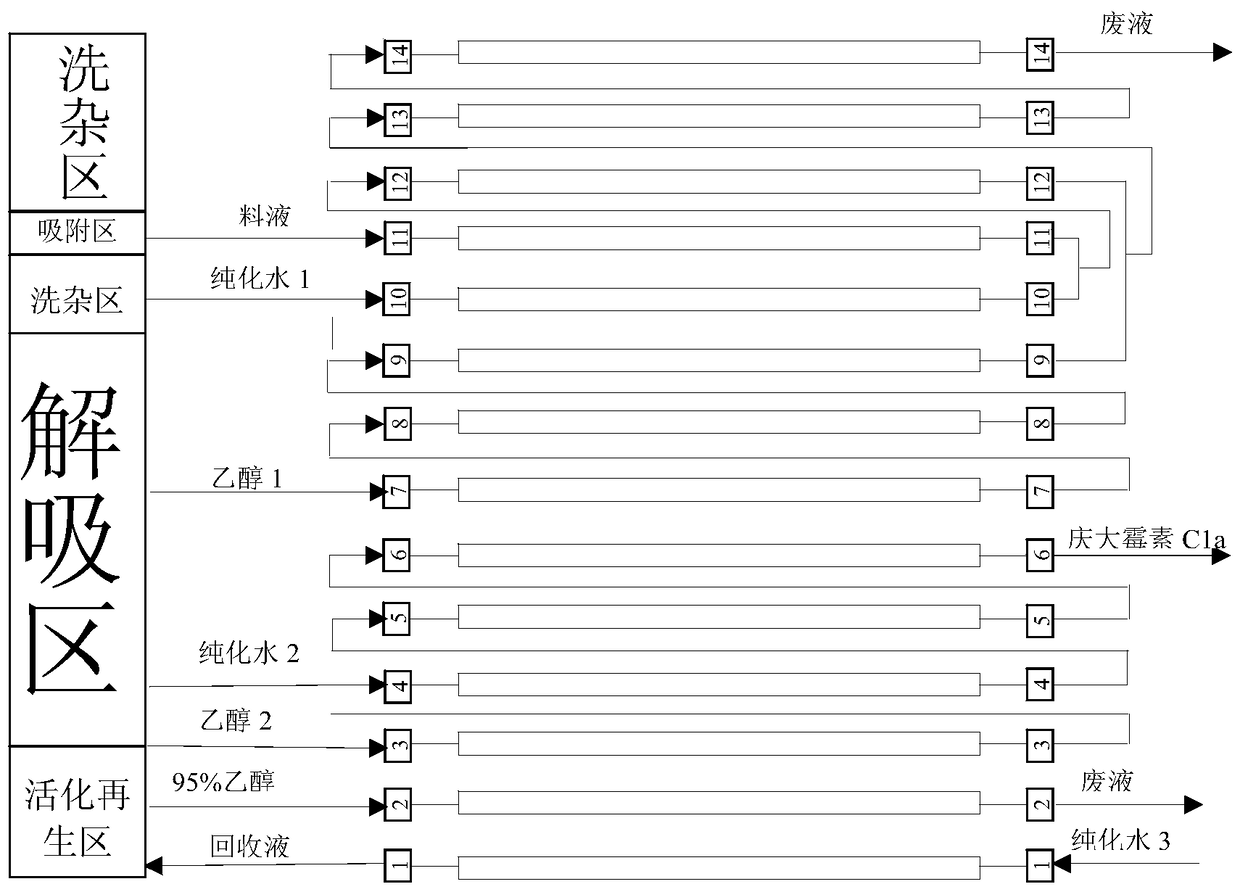
Continuous chromatographic separation technology of gentamycin C1a
ActiveCN105524128AReduce layoutSmall footprintSugar derivativesChemical recyclingChromatographic separationGentamicin C1a
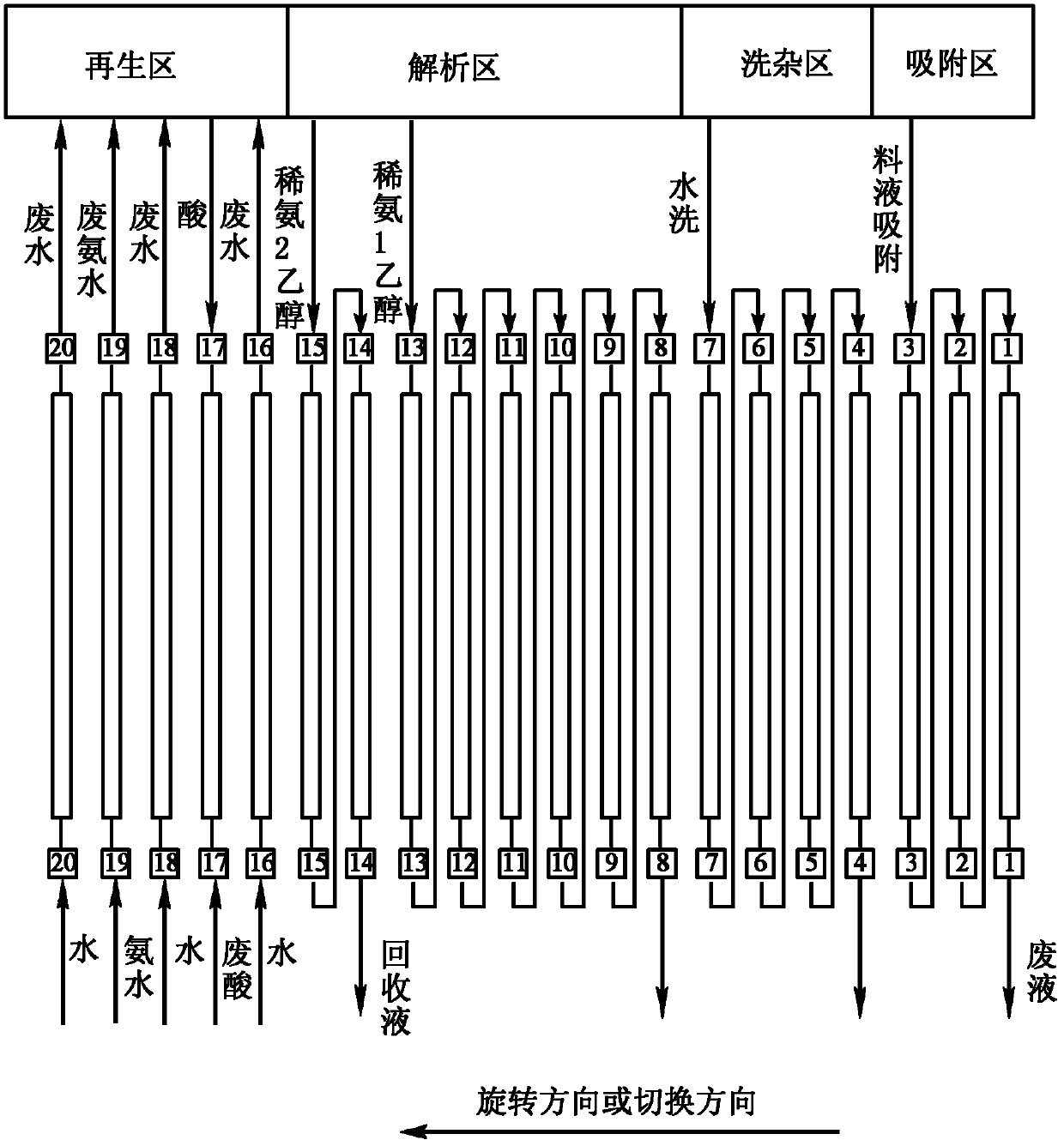
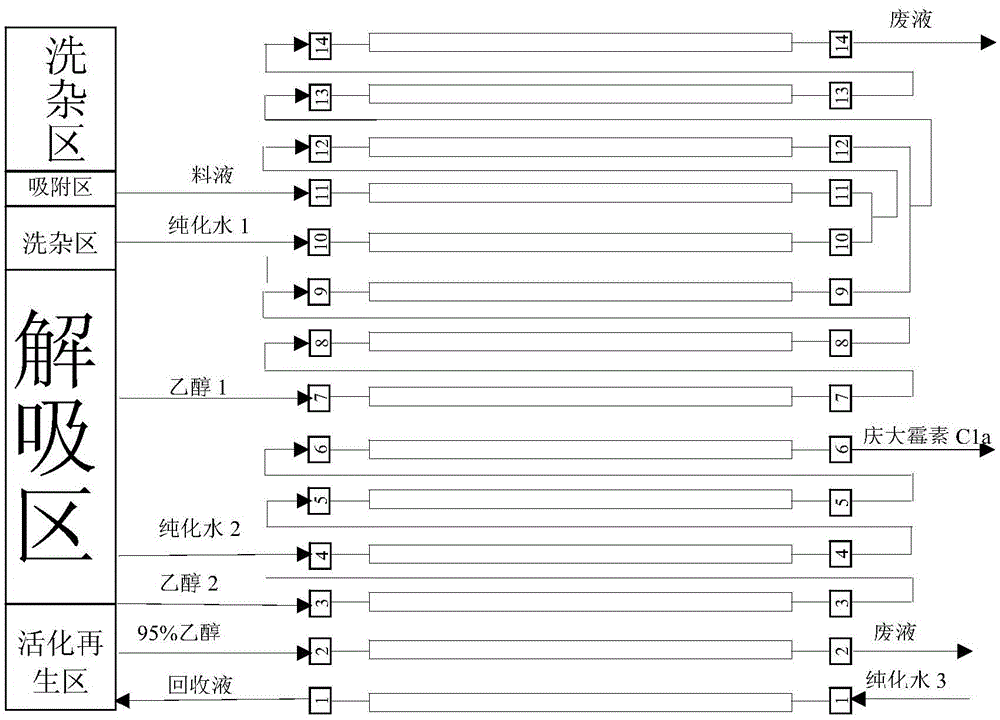
The invention relates to a continuous separation and purification technology of gentamycin C1a. Efficient separation of a gentamycin C1a recovered liquid is realized through using a continuous chromatographic separation technology. The technology comprises the following steps: allowing recovered gentamycin C1a to enter a continuous chromatographic system, adsorbing, washing out impurities, eluting, collecting the obtained eluate, regenerating a chromatographic column, and concentrating the collected eluate to obtain gentamycin C1a. The gentamycin C1a separated in through the technology has the advantages of high yield and high purity, so the technology has the advantages of low cost, environmental protection and suitableness for industrial production.
Owner:WUXI JIMIN KEXIN SHANHE PHARMA +1
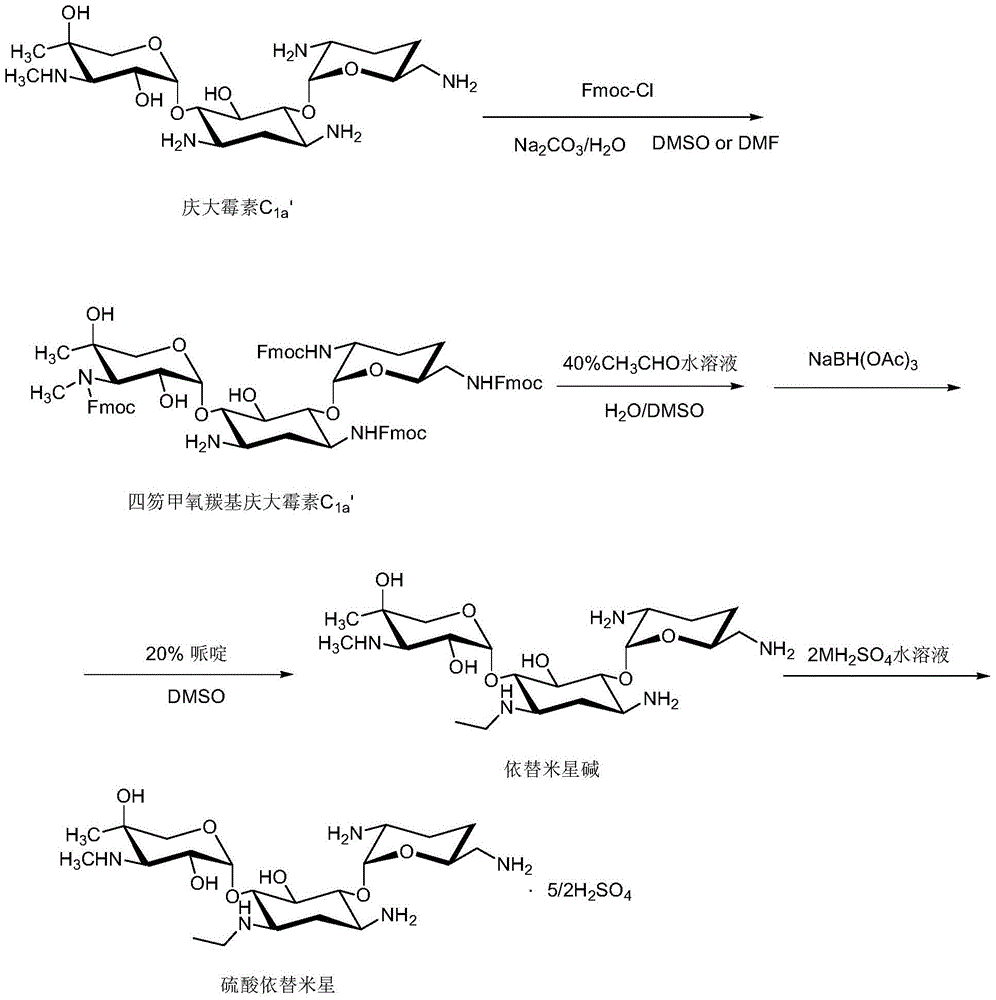
Etimicin sulfate preparation method
ActiveCN104231016ALow impurity contentAcid environment stableSugar derivativesSugar derivatives preparationSodium triacetoxyborohydrideGentamicin C1a
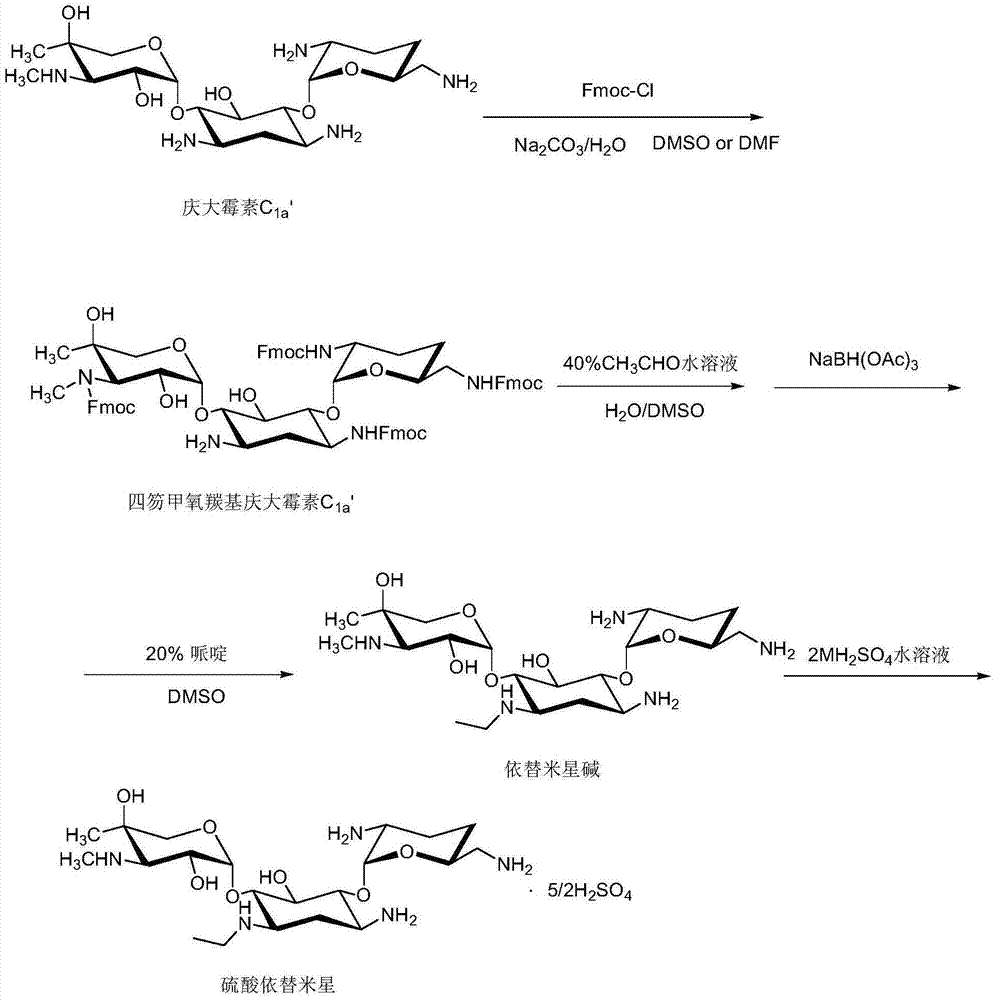
The invention discloses an etimicin sulfate preparation method. The etimicin sulfate preparation method includes the steps of protecting amino groups at 3, 2', 6' and 3' of gentamicin C1a with Fmoc-Cl and performing purification and evaporation to dryness to obtain quadrifluorenyl methoxycarbonyl gentamicin C1a; adding N-ethyl of an acetaldehyde aqueous solution under acidic conditions and performing reduction with sodium triacetoxyborohydride; finally adding a piperidine / DMSO (dimethylsulfoxide) solution for deprotection; performing secondary purification on an reaction solution with weakly acidic cation adsorption resin after performing primary purification on the reaction solution with a macroporous adsorption resin column and adding sulfuric acid to a purified product to obtain etimicin sulfate through reaction. The etimicin sulfate preparation method is high in selectivity and less in side reaction in group protecting, mild in reaction conditions, simple in operation and easy to perform industrial production during the whole reaction process and high in purity and yield of the product. (An equation as shown in the description).
Owner:QILU PHARMA HAINAN +1
Preparation method of gentamicin sulphate C1a
InactiveCN104017844ARaise the fermentation unitSimple production processMicroorganism based processesFermentationBiotechnologyGentamicin C1a
The invention the technical field of antibiotics fermentation, and provides a preparation method of gentamicin sulphate C1a. According to the preparation method, the output of gentamicin C1a is increased by improving the component dosage of a gentamicin fermentation culture medium. The preparation method of the gentamicin sulphate C1a is simple in production process and easy to operate and obtains the high-quality gentamicin sulphate C1a by improving the component dosage and extraction and separation method of the gentamicin sulphate fermentation culture medium.
Owner:福安药业集团烟台只楚药业有限公司
Technological method for producing gentamicin C1a through fermentation
PendingCN106801076AImprove metabolism and synthesis abilityIncrease productionBacteriaMicroorganism based processesSporeBiotechnology
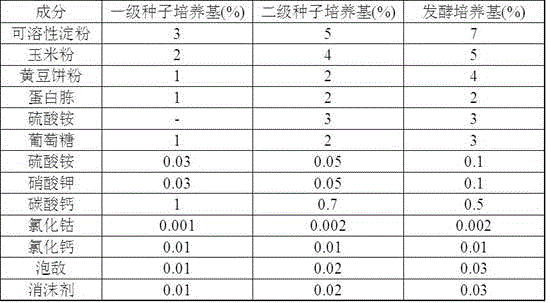
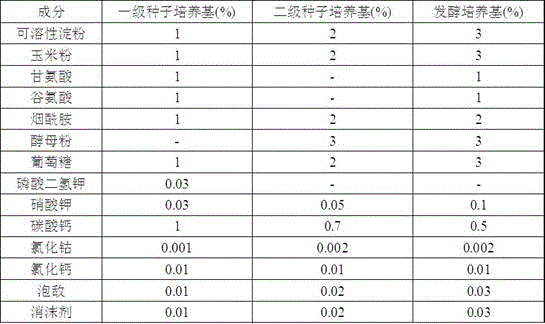
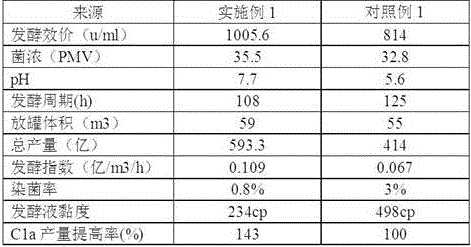
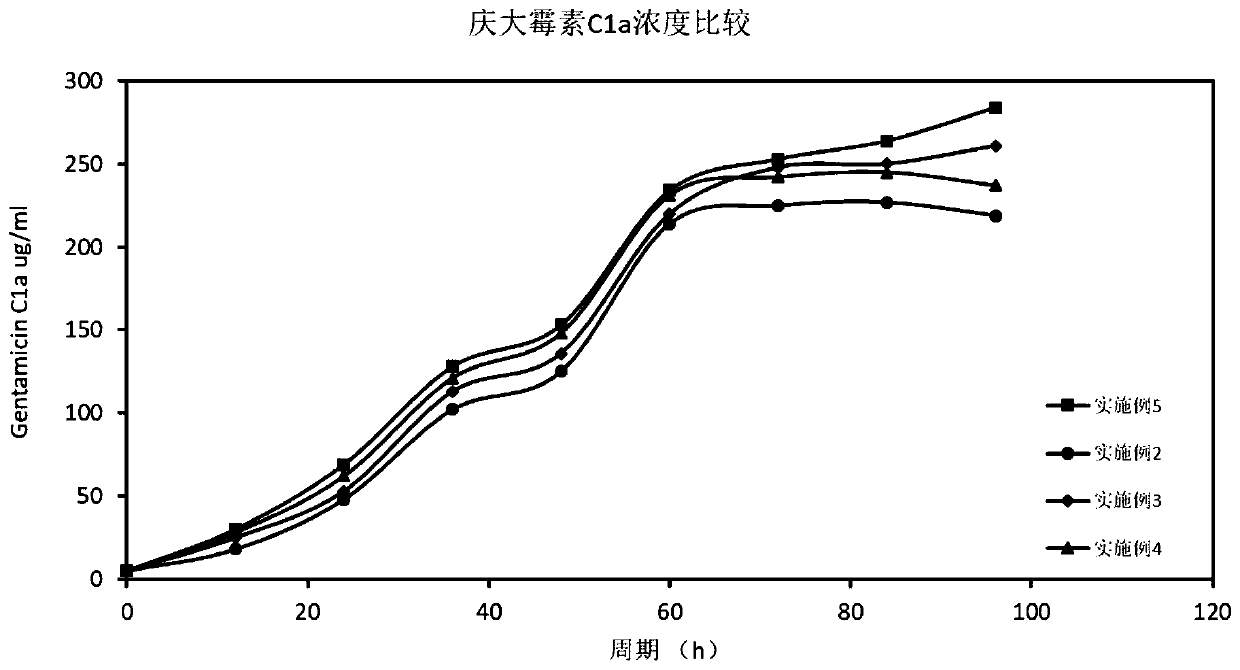
The invention relates to a technological method for producing gentamicin C1a through fermentation, and belongs to the technical field of fermentation engineering. The technological method for producing gentamicin C1a through fermentation comprises the following concentrated technological means: spore culture, seed liquid preparation, primary seed culture, secondary seed culture, fermentation production and material supplementation control, wherein components and proportions of a primary seed culture medium, a secondary seed culture medium and a fermentation culture medium are mainly provided by the technological method; meanwhile, in the material supplementation control stage, sugar supplementing, water supplementing, defoamer supplementing, alkali supplementing and nitrogen supplementing operations are performed, and the material supplementing time, the material supplementing components and the material supplementing amount are controlled. The technological method is achieved by relying on culture media for producing the gentamicin C1a through efficient and low-cost fermentation, and in the fermentation process, technological control is performed, so that the fermentation titer unit is improved and the yield is increased by 43%; meanwhile, the production cost is reduced to the maximum extent; therefore, the technological method is very practical during production of the gentamicin C1a.
Owner:HENAN RENHUA BIOTECH CO LTD
Method for preparing Etimicin sulfate
InactiveCN102250166AShorten the synthesis processEmission reductionSugar derivativesSugar derivatives preparationGentamicin C1aSlag
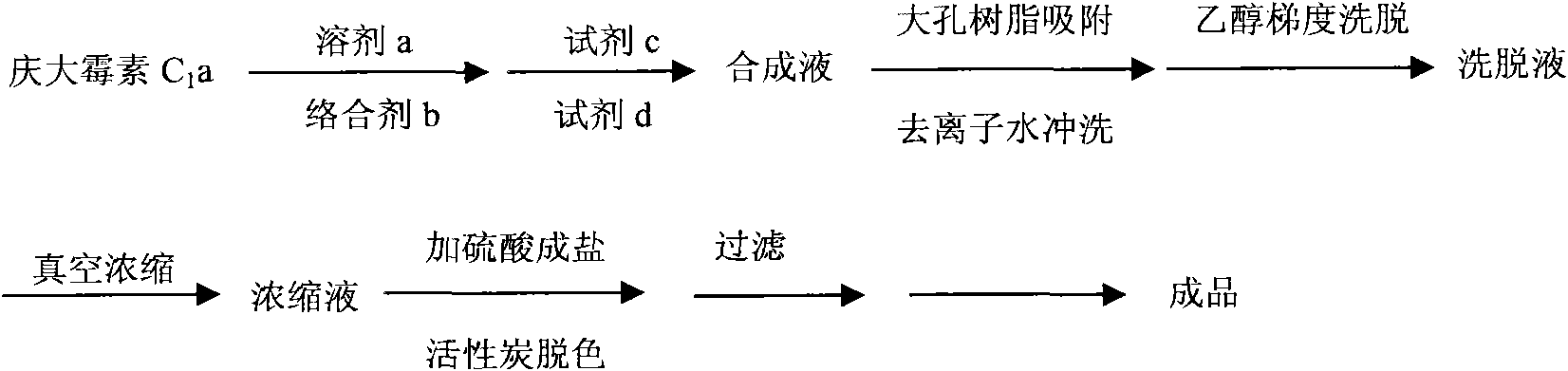
The invention relates to a method for preparing Etimicin sulfate. In the method, under the action of a complex protective agent, gentamicin Cla is subjected to 1-N-ethylation, the synthesized product is separated by macroporous resin, the synthesized product is concentrated, the pH value of the synthesized product is adjusted by sulfuric acid to form a salt, the salt is decolorized by active carbon and freeze-dried; and the medicinal Etimicin sulfate meeting related standards is obtained. When the method is used for producing Etimicin sulfate, the synthesis process can be shortened greatly, the labor production efficiency is improved, and the discharge of waste water, gas and slag is reduced.
Owner:JIANGXI JEMINCARE GRP CO LTD +1
Method for preparing etimicin sulfate
ActiveCN103113430AReduce generationHigh selectivitySugar derivativesSugar derivatives preparationReaction temperatureSide reaction
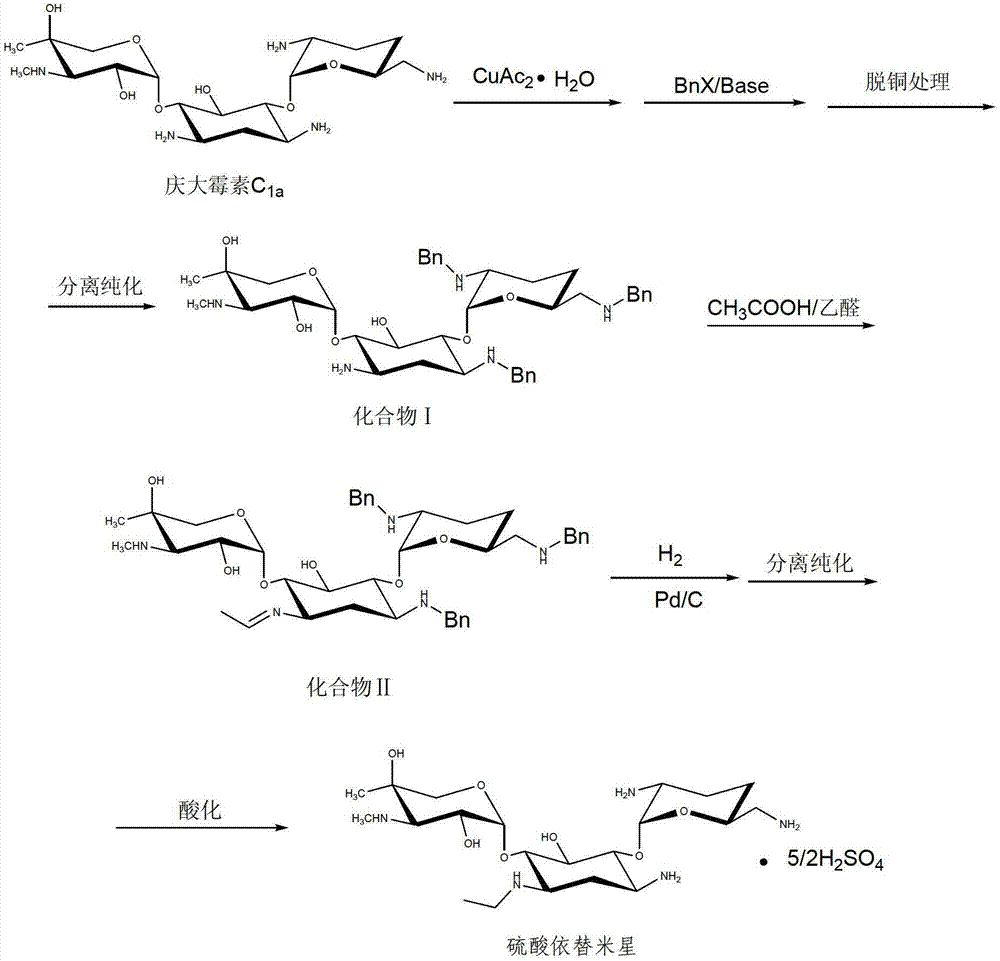
The invention discloses a method for preparing etimicin sulfate, and belongs to the technical field of medicines. The method comprises the steps of: adding copper acetate monohydrate into gentamicin C1a to form a copper ion complex; then adding a benzyl compound into a reaction system, controlling the temperature at 15-40 DEG C, and then adding alkali into the reaction system to react; decoppering and purifying the obtained reaction liquid so as to obtain a light yellow oily compound I; adding acetic acid into the oily substance, controlling the temperature at 5-15 DEG C, adding acetaldehyde to react for a while, adding a catalyst Pd / C and continuously filling hydrogen to react; and separating, purifying and acidizing the reaction liquid so as to obtain etimicin sulfate. The method of utilizing benzyl to protect amino groups is adopted, and high selectivity is obtained; and two reaction steps of reduction and deprotection are completed through a 'one-pot method', and the method is low in reaction temperature and takes a short period, thus, side reaction is less, purification is easy, the reaction and separation costs are lowered, and the method can be easily applied to industrial production.
Owner:QILU PHARMA HAINAN +1
Preparation method for gentamicin C1a
InactiveCN106498011AStable outputReduce manufacturing costMutant preparationMicroorganism based processesFiltration membraneGentamicin B
The invention provides a preparation method for gentamicin C1a. The preparation method comprises the following steps: performing the microwave mutagenesis and chemical mutagenesis to gentamicin B producing bacteria, selecting a new bacterial strain in which the gentamicin C1a is the main component, performing the microwave mutagenesis firstly and performing the chemical mutagenesis secondly to obtain the producing bacteria of the gentamicin C1a, performing the fermenting cultivation, separating the obtained fermentation liquor through a micro-filtration membrane, an ultra-filtration membrane and a nano-filtration membrane so as to obtain a crude product of the gentamicin C1a. The yield of the gentamicin C1a is very stable through the continuous passage of the producing bacteria of the obtained gentamicin C1a, the production cost is lower, and the preparation method is suitable for the industrialization application.
Owner:WUXI FORTUNE PHARMA
Method for preparing 3,2',6'-tri-N-acetyl gentamicin C1a
ActiveCN101928310AReduce repulsionHigh yieldSugar derivativesSugar derivatives preparationAcetic acidAcetic anhydride
The invention discloses a method for preparing 3,2',6'-tri-N-acetyl gentamicin C1a, which comprises the following steps of: (1) performing coordination reaction on gentamicin C1a and zinc acetate in a methanol solvent at the temperature of between 15 and 25 DEG C to prepare a gentamicin C1a-Zn complex; (2) reducing the temperature of the system of the step (1) to between 0 and 10 DEG C, dropwise adding mixed liquid consisting of acetic anhydride, triethylamine and tetrahydrofuran with stirring for acylation reaction to prepare 3,2',6'-tri-N-acetyl gentamicin C1a-Zn complex, after dropwise adding, continuously stirring and reacting for 1 to 2 hours, and adding water and distilling under reduce pressure to prepare concentrated solution containing the 3,2',6'-tri-N-acetyl gentamicin C1a-Zn complex; and (3) performing after treatment on the concentrated solution prepared by the step (2) to prepare a 3,2',6'-tri-N-acetyl gentamicin C1a finished product. The method has the advantage of higher yield.
Owner:CHANGZHOU FANGYUAN PHARMA +1
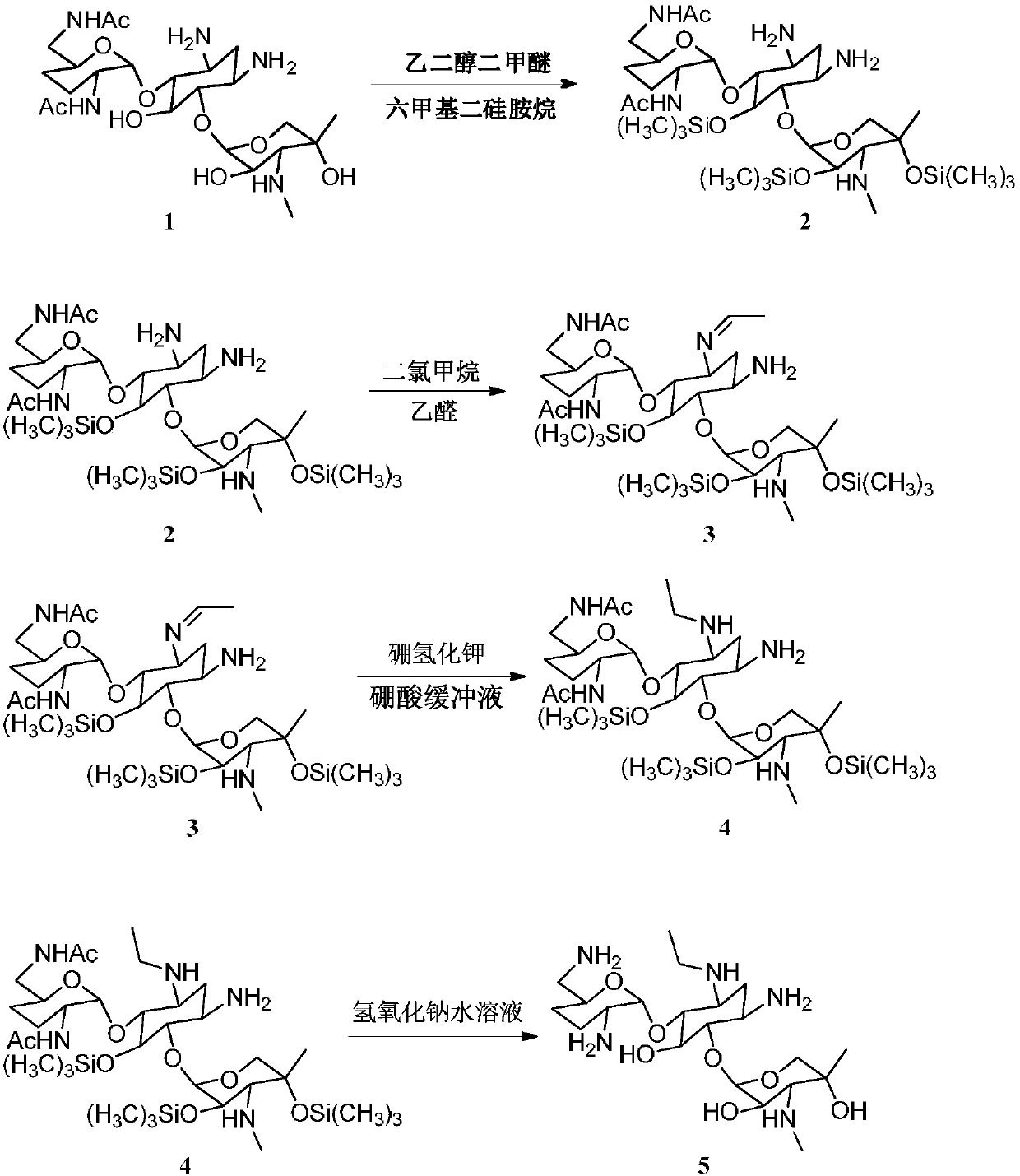
Synthesizing method of 3-N-ethyl gentamicin C1a
InactiveCN107652334AHigh purityHigh yieldSugar derivativesSugar derivatives preparationPotassium borohydridePotassium
The present invention relates to a kind of synthetic method of 3-N-ethyl gentamicin C1a, the method comprises the following steps: ethylene glycol dimethyl ether, hexamethyldisilazane, concentrated sulfuric acid, 2 ", 6" ‑N,N‑Diacetylgentamycin C1a was put into a round-bottomed flask and refluxed. After the reactants were dissolved, part of the solvent was distilled off. Dichloromethane was added and stirred at 10°C, and acetaldehyde was added dropwise. After the reaction was completed, potassium borohydride and boric acid buffer were added to stir the reaction, and part of the solvent was distilled off under normal pressure. Add 10%-20% sodium hydroxide solution, heat to reflux, cool down to room temperature, and concentrate the reaction system. Desalting, the target compound 3-N-ethyl gentamycin C1a was obtained by separation.
Owner:WUXI JIMIN KEXIN SHANHE PHARMA +1
Etimicin sulfate preparation method
ActiveCN105524129AEasy to operateEasy to industrializeSugar derivativesSugar derivatives preparationGentamicin C1aAcetylation
The invention provides an etimicin sulfate preparation method. The method comprises the following steps: taking gentamycin C1a base, dissolving the gentamycin C1a base in a certain proportion of a solvent, adding a certain proportion of a complexing agent to complex, adding a certain proportion of an amino group protection agent, keeping for a certain time, adding a certain proportion of a precipitating agent, stirring above materials, filtering the obtained mixture, adding a certain proportion of an acetylation reagent to the above obtained filtrate, reacting for a certain time, adding a certain proportion of a reducing agent, reacting for a certain time, removing an amino protection group, adding a certain proportion of water, carring out vacuum concentration under certain conditions to remove the solvent, adjusting the pH value with ammonia water, adsorbing with macro-porous resin, carrying out gradient separating purification with diluted ethanol, collecting an etimicin solution with a certain purity, carrying out vacuum concentration under certain conditions, adding sulfuric acid to adjust the pH value, adding a certain proportion of active carbon, decolorizing, filtering, and drying to obtain etimicin sulfate according with relevant standards.
Owner:WUXI JIMIN KEXIN SHANHE PHARMA +1
Preparation method of etimicin sulfate
InactiveCN108409814AImprove solubilityImprove quality controlSugar derivativesSugar derivatives preparationPalladium on carbonAcetic acid
The invention relates to a preparation method of etimicin sulfate. The preparation method includes following steps: taking gentamicin C1a base, dissolving in a solvent of a certain proportion, addinga complexing agent of a certain proportion, adding an amino protection agent of a certain proportion, maintaining for certain time, adding a solvent and ammonia water of a certain proportion, stirring, skimming, and concentrating to obtain a first intermediate; taking a reducing agent of a certain proportion, adding a solvent and acetic acid of a certain proportion, maintaining for certain time, adding the first intermediate, allowing reaction for a period of time, adding a strong alkaline solution to adjust pH, filtering, and concentrating to obtain a second intermediate; adding a solvent anda palladium carbon catalyst of a certain proportion, allowing reaction at certain hydrogen pressure and temperature, filtering, concentrating, using resin for adsorption, using ammonia water for resolving, concentrating, using sulfuric acid to adjust pH, removing carbon, filtering, and drying to obtain etimicin sulfate.
Owner:NINGBO TEAM PHARMA
Synthesis method of etimicin sulfate intermediate (3,2',6'-tri-N-acetyl gentamicin C1a)
ActiveCN103524577ARelieve pressureReduce usageSugar derivativesSugar derivatives preparationChromatographic separationAcetic anhydride
The invention relates to a synthesis method of an etimicin sulfate intermediate (3,2',6'-tri-N-acetyl gentamicin C1a). The synthesis method comprises the following steps: a, adding 20 L of methanol, 2.70 kg of gentamicin C1a and 3.21 kg of anhydrous zinc acetate into a 50 L three-port bottle at a temperature of 29 DEG C, stirring for 1.5 h to dissolve, and thus obtaining a gentamicin C1a-Zn complex; b, dripping 2.55 L of acetic anhydride into the complex of the step a for acetylation; c, after finishing the reaction, concentrating the reaction liquid of the step b, introducing the concentrated liquid into a chromatographic separation column for charging the sample, rinsing with a salt-free water, resolving with an ethanol water solution, and collecting effective components; concentrating, drying to obtain 3.29 kg of the finished product P1, wherein the yield is 95.3%, and the purity is 96.1%.
Owner:WUXI JIMIN KEXIN SHANHE PHARMA +1
Synthesis method of 6''-N-ethyl gentamicin C1a
ActiveCN110498823AStrong specificityLess side effectsSugar derivativesSugar derivatives preparationAcetic anhydridePotassium borohydride
The invention relates to a synthesis method of 6''-N-ethyl gentamicin C1a. The synthesis method of the 6''-N-ethyl gentamicin C1a comprises the following steps that (1) a compound 1 gentamicin C1a isdissolved in a solvent for cooling, BOC-ON is added and stirred, and water and ethyl acetate are added to be subjected to liquid separating, water phase taking, and condensing to obtain a compound 2;(2) a concentrated solution is dissolved by methanol, triethylamine is added for cooling, and acetic anhydride is added, stirred and condensed to remove a solvent; (3) hydrochloric acid is added to aconcentrated solution, and stirred at the room temperature, pH is adjusted by a sodium hydroxide solution for concentration, and a compound 3, namely 1,3,2''-N,N,N-triacetyl gentamicin C1a is obtainedby purification and separation through a silica gel column; and (4) after the 1,3,2''-N,N,N-tracetyl gentamicin C1a, glycol dimethyl ether, hexamethyldisilazane and a concentrated sulfuric acid are mixed and heated until refluxing and dissolving, stirring and concentrating continue to be conducted to remove the solvent so as to obtain a compound 4, dichloromethane is added for cooling, acetaldehyde is added to be stirred, potassium borohydride is added to be stirred, a borate buffer solution is added to be stirred, sodium hydroxide is added for condensing and removing the dichloromethane, thesodium hydroxide is added for heating and refluxing, and macroporous resin is subjected to desalination and separation to obtain a compound 5, namely 6''-N- ethyl gentamicin C1a.
Owner:WUXI JIYU SHANHE PHARM CO LTD
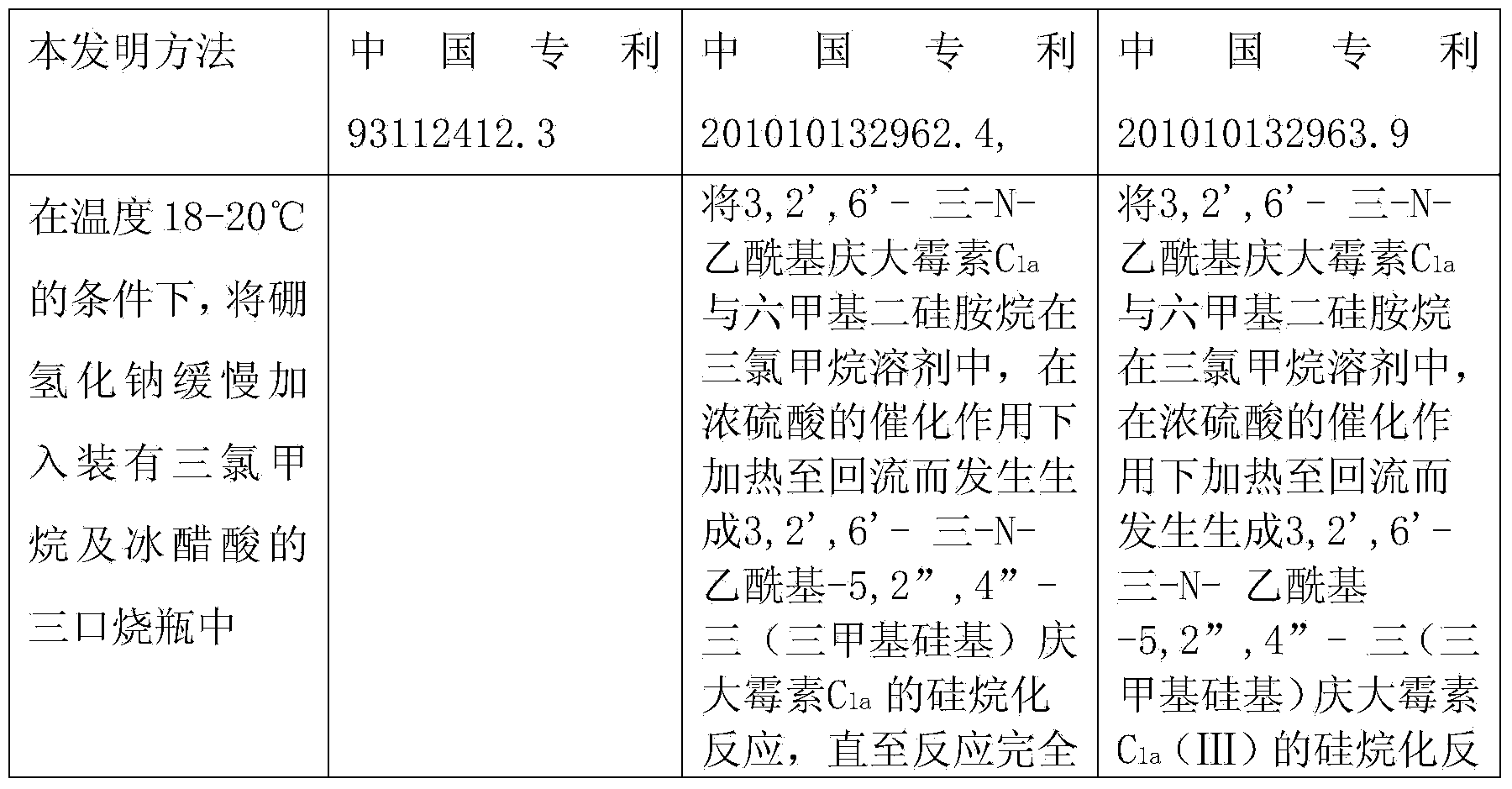
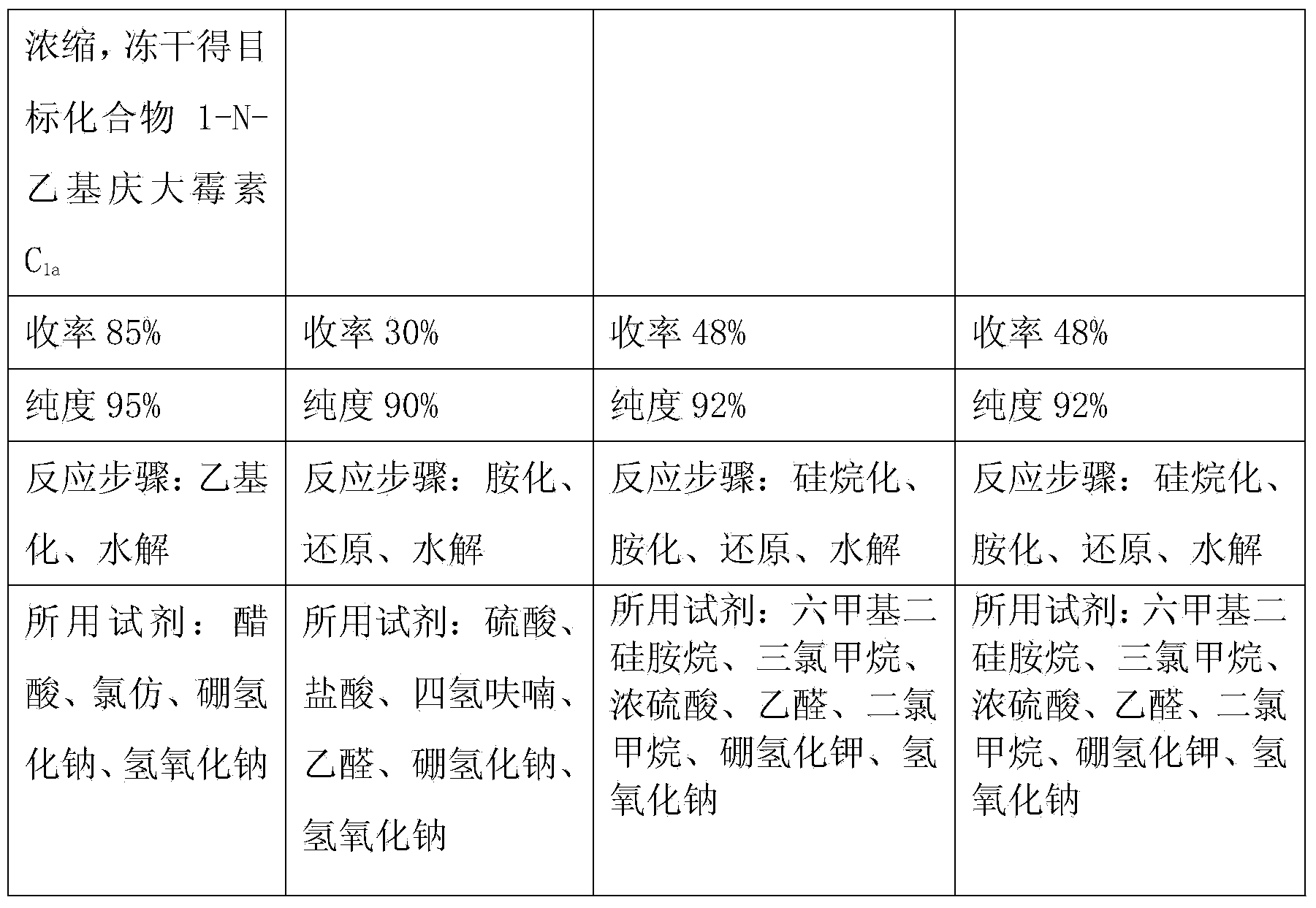
Preparation method of 1-N-ethyl gentamicin C1a
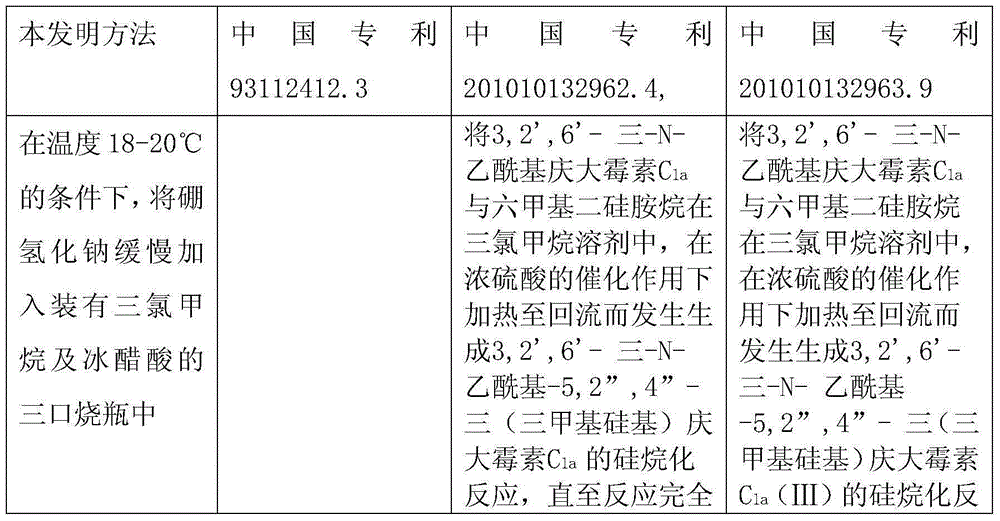
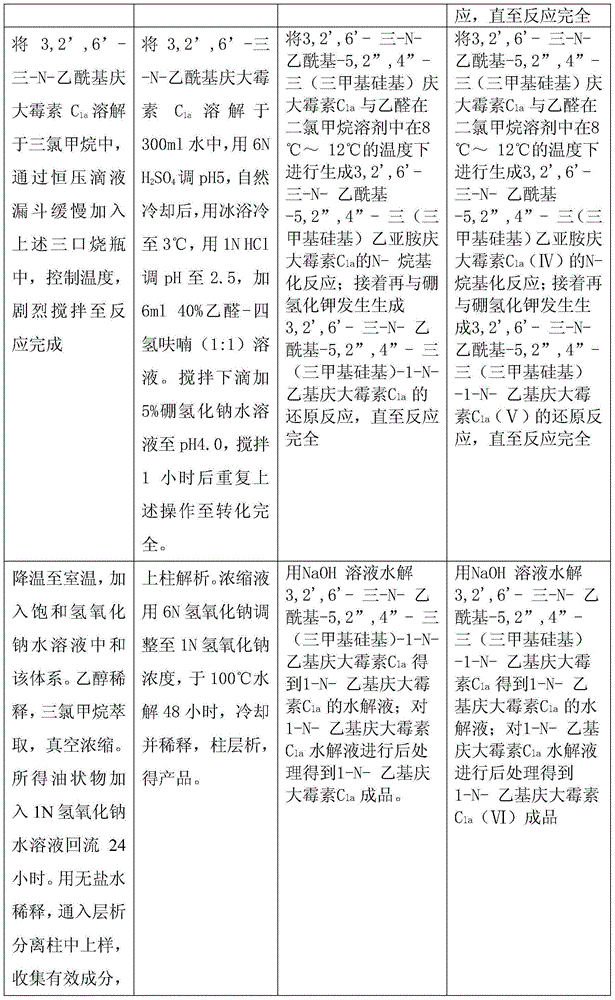
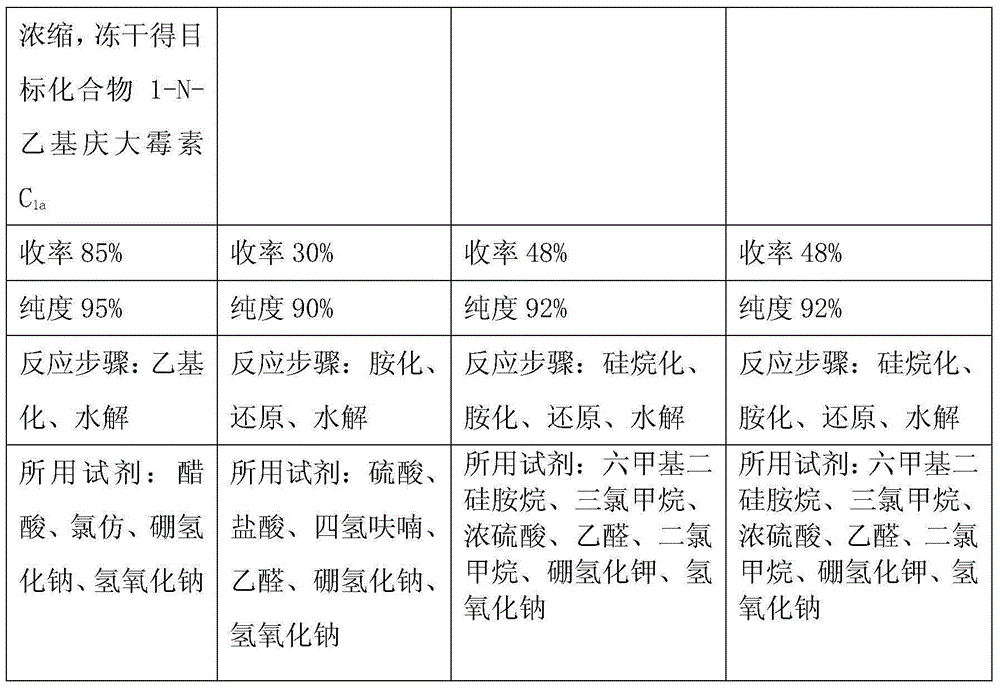
The invention relates to a preparation method of 1-N-ethyl gentamicin C1a. The method comprises the following steps: under the condition of 18-20 DEG C, slowly adding sodium borohydride into three flasks with trichloromethane and glacial acetic acid, dissolving 3,2',6'-tri-N-gentamicin C1a into trichloromethane, slowly adding the mixture into the flasks through a constant pressure dropping funnel, keeping the temperature to 40-50 DEG C, vigorously stirring for reaction 20 hours, cooling and adding a saturated sodium hydroxide aqueous solution to neutralize the system, diluting with ethyl alcohol, extracting with trichloromethane, performing vacuum concentration to obtain the oily matter, and adding a 1N sodium hydroxide aqueous solution for circulation reflux for 24 hours; diluting with salt-free water, performing column chromatography isolation, collecting effective constituents, concentrating and freeze-drying to obtain 1-N-ethyl gentamicin C1a.
Owner:WUXI JIMIN KEXIN SHANHE PHARMA +1
Method for detecting gentamicin C1a
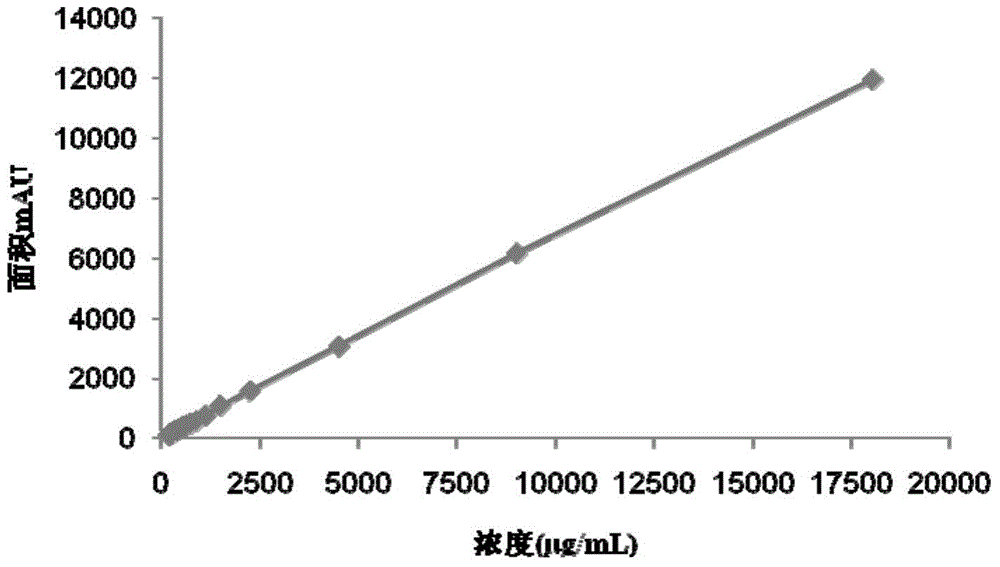
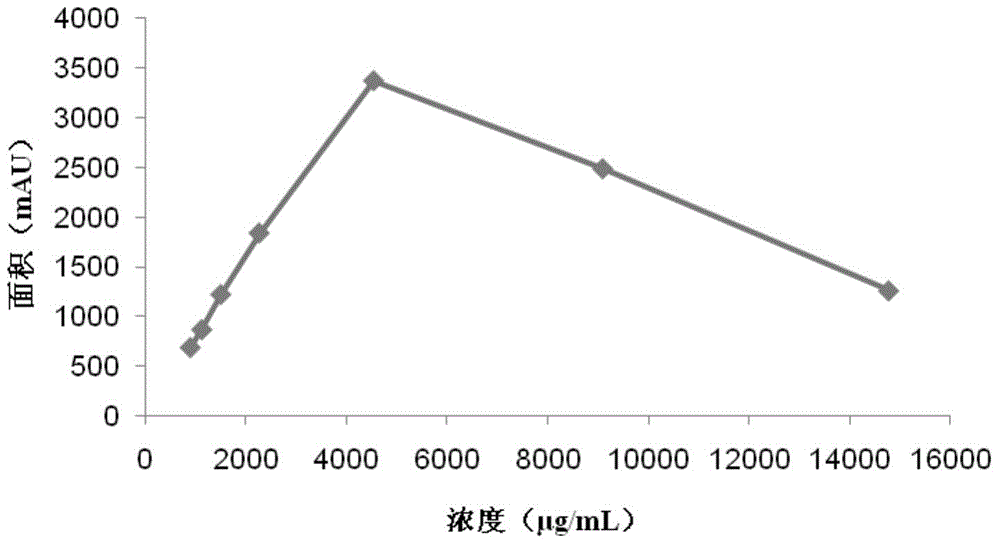
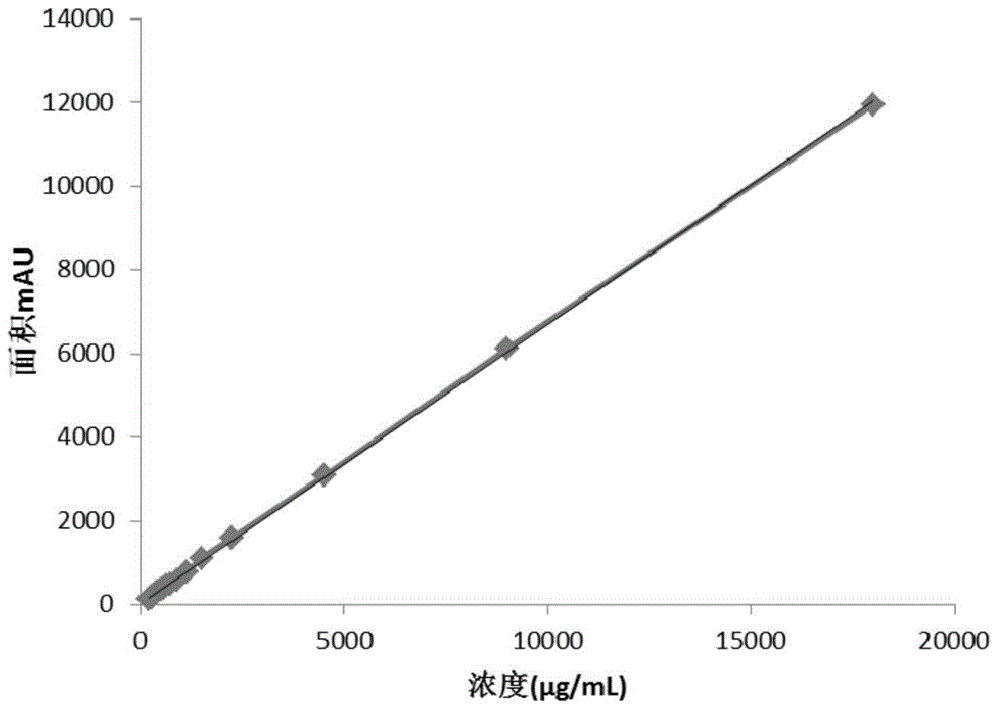
The invention discloses a method for detecting gentamicin C1a, wherein the method comprises the following steps: mixing a to-be-detected sample containing gentamicin C1a with a derivatization reagent evenly and carrying out a derivatization reaction, and then detecting by using a liquid chromatography or liquid chromatography-mass spectrometry detection method. The derivatization reagent contains several components of a solvent, 1,2-phthalic dicarboxaldehyde, mercaptoacetic acid and a buffer solution; the molar ratio of 1,2-phthalic dicarboxaldehyde to mercaptoacetic acid in the derivatization reagent is 1:3.8-1:0.5; the pH of the derivatization reagent is 10-11; the ratio of the molar amount of 1,2-phthalic dicarboxaldehyde in the derivatization reagent to the molar amount of gentamicin C1a detected in the to-be-detected sample in a single detection result is greater than or equal to 3.59. The method for detecting gentamicin C1a has the advantages of simple operation, and relatively high accuracy and wide linear range in detection of the gentamicin C1a concentration in production samples.
Owner:益诺思生物技术南通有限公司
A kind of synthetic method of etimicin sulfate
ActiveCN103833804BHigh yieldSugar derivativesSugar derivatives preparationAcetic anhydrideEthylic acid
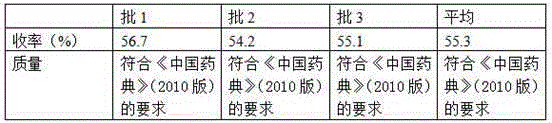
The invention discloses a synthetic method of etimicin sulfate. The synthetic method comprises the following steps: carrying out a reaction on gentamicin C1a, zinc acetate and acetic anhydride to obtain 3, 2', 6'-tri(N-acetyl) gentamicin C1a; after separating and purifying the 3, 2', 6-tri(N-acetyl) gentamicin C1a, carrying out a reaction with iodoethane to obtain 3, 2', 6'-tri(N-acetyl)-1-ethyl gentamicin C1a; hydrolyzing the 3, 2', 6'-tri(N-acetyl)-1-ethyl gentamicin C1a by sodium hydroxide to obtain an aqueous solution of etimicin sulfate; and carrying out HZD-2 macroporous resin adsorption, separation and purification, concentration, sulfate formation, decarburization and drying on the aqueous solution of etimicin sulfate to obtain an etimicin sulfate finished product. The method disclosed by the invention is high in yield which is 55%, and satisfies the requirements of Chinese pharmacopoeia (Edition 2010).
Owner:福安药业集团烟台只楚药业有限公司
A kind of continuous chromatographic separation process of gentamicin c1a
ActiveCN105524128BReduce layoutSmall footprintSugar derivativesChemical recyclingChromatographic separationGentamicin C1a
Owner:WUXI JIYU SHANHE PHARM CO LTD +1
A kind of preparation method of etimicin sulfate
ActiveCN104231016BLow impurity contentAcid environment stableSugar derivativesSugar derivatives preparationGentamicin C1aSodium triacetoxyborohydride
The invention discloses an etimicin sulfate preparation method. The etimicin sulfate preparation method includes the steps of protecting amino groups at 3, 2', 6' and 3' of gentamicin C1a with Fmoc-Cl and performing purification and evaporation to dryness to obtain quadrifluorenyl methoxycarbonyl gentamicin C1a; adding N-ethyl of an acetaldehyde aqueous solution under acidic conditions and performing reduction with sodium triacetoxyborohydride; finally adding a piperidine / DMSO (dimethylsulfoxide) solution for deprotection; performing secondary purification on an reaction solution with weakly acidic cation adsorption resin after performing primary purification on the reaction solution with a macroporous adsorption resin column and adding sulfuric acid to a purified product to obtain etimicin sulfate through reaction. The etimicin sulfate preparation method is high in selectivity and less in side reaction in group protecting, mild in reaction conditions, simple in operation and easy to perform industrial production during the whole reaction process and high in purity and yield of the product. (An equation as shown in the description).
Owner:QILU PHARMA HAINAN +1
Method for preparing 3,2',6'-tri-N-acetyl gentamicin C1a
ActiveCN101928310BReduce repulsionHigh yieldSugar derivativesSugar derivatives preparationAcetic acidAcetic anhydride
Owner:CHANGZHOU FANGYUAN PHARMA +1
Purification method of gentamicin C1a
ActiveCN110563782AAchieve purificationHigh puritySugar derivativesSugar derivatives preparationPurification methodsAlcohol
The invention discloses a purification method of gentamicin C1a. The method comprises the following steps: (1) separation by macroporous adsorption resin column chromatography; and (2) salting out crystallization: mixing methanol and ethanol to obtain a mixed alcohol, mixing the mixed alcohol with the analytical solution of step (1) according to a volume ratio of 5:1-7:1, conducting magnetic stirring at 20-25 DEG C, a solid being precipitated, and the precipitated solid being purified gentamicin C1a. According to the present invention, a method of combining macroporous adsorption resin columnchromatography and salting out crystallization is adopted to achieve the purification of gentamicin C1a, the purity of gentamicin C1a is raised to 98% or above, and gentamicin C1a with high purity isobtained. The purification method of the invention has the advantages of simple steps, good purification effect, and high purification yield of gentamicin C1a.
Owner:CHANGZHOU FANGYUAN PHARMA +2
Preparation method of 1-N-ethyl gentamicin C1a sulfate
ActiveCN101928312BReduce repulsionHigh yieldSugar derivativesSugar derivatives preparationWater methanolPotassium borohydride
The invention discloses a preparation method of 1-N-ethyl gentamicin C1a sulfate. The method comprises the following steps of: carrying out a complexation reaction on gentamicin C1a and zinc acetate in a methanol solvent; then, dropping a mixed solution of acetic anhydride, triethylamine and tetrahydrofuran to carry out an acylation reaction and obtaining 3,2',6'-3-N-acetyl-gentamicin C1a by post-treatment; then, carrying out a silylation reaction with hexamethyl disilazane in a chloroform solvent; carrying out an N-alkylation reaction with acetaldehyde in a carrene solvent; then, carrying out a reduction reaction with potassium borohydride; hydrolyzing with an NaOH solution; obtaining 1-N-ethyl gentamicin C1a by post-treatment; adding the 1-N-ethyl gentamicin C1a to anhydrous ethanol or anhydrous methanol for stirring or dissolving; then, dropping concentrated sulfuric acid; and obtaining 1-N-ethyl gentamicin C1a sulfate by post-treatment. The method of the invention has higher yield.
Owner:CHANGZHOU FANGYUAN PHARMA +1
Detection method of gentamicin C1a
The invention discloses a detection method of gentamicin C1a. The method comprises the following steps: evenly mixing a gentamicin C1a-containing sample to be tested with a derivatization reagent, and carrying out a derivatization reaction; after that, detecting by a method of liquid chromatography or liquid chromatography-mass spectrometry, wherein the derivatization reagent contains the following components: a solvent, phthalic dicarboxaldehyde, mercaptoacetic acid and a buffer solution; the molar ratio of the phthalic dicarboxaldehyde to the mercaptoacetic acid in the derivatization reagent is (1to 8)-(1to 3.89); the pH value of the derivatization reagent is equal to 10-11; the ratio of the molar quantity of the phthalic dicarboxaldehyde in the derivatization reagent used in a single detection result to the molar quantity of the gentamicin C1a obtained by testing the sample to be tested needs to be more than or equal to 1.6. The detection method of the gentamicin C1a is simple in operation; the detection method is higher in accuracy and wide in linear range when being used for detecting the concentration of the gentamicin C1a in a production sample.
Owner:SHANGHAI INST OF PHARMA IND +1
Synthetic medium as well as preparation method and use thereof
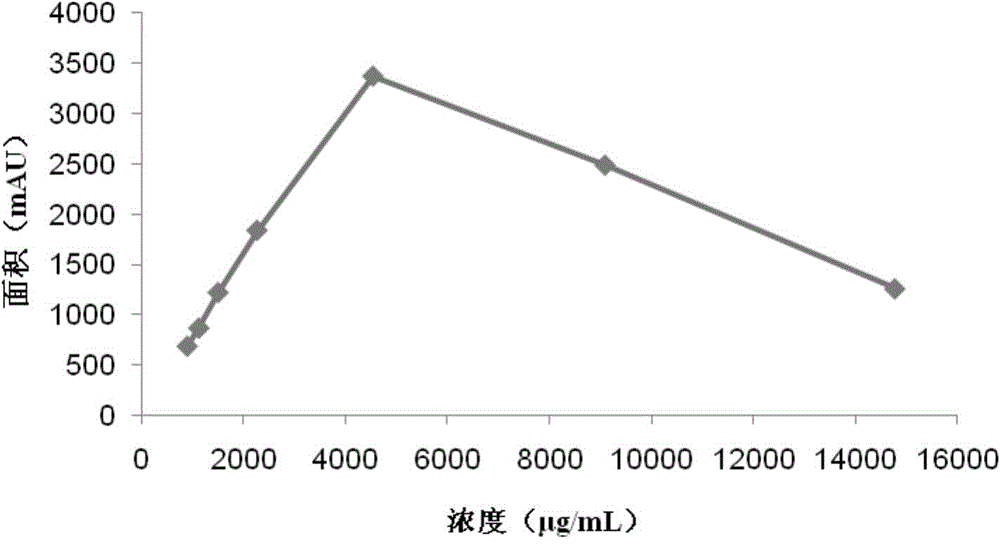
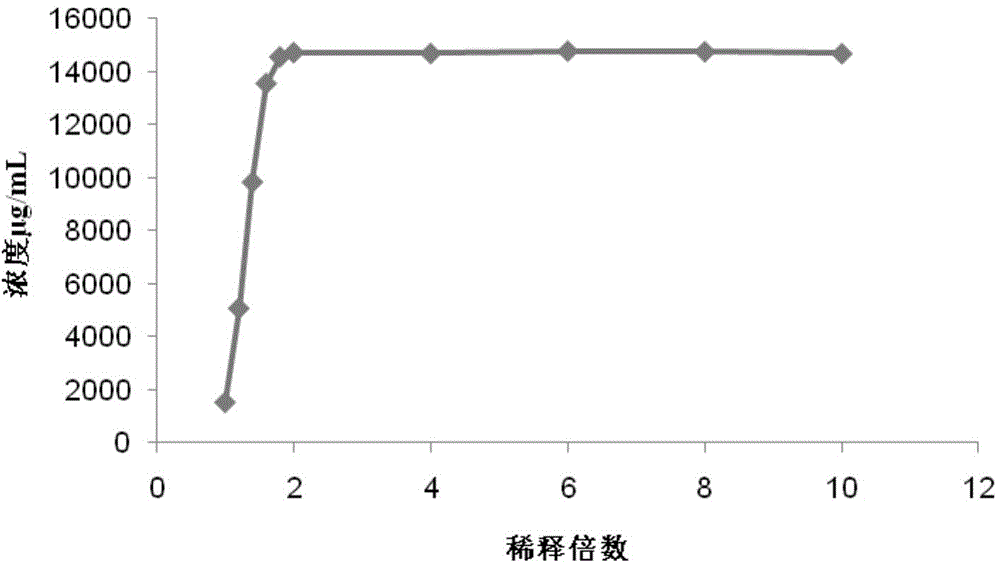
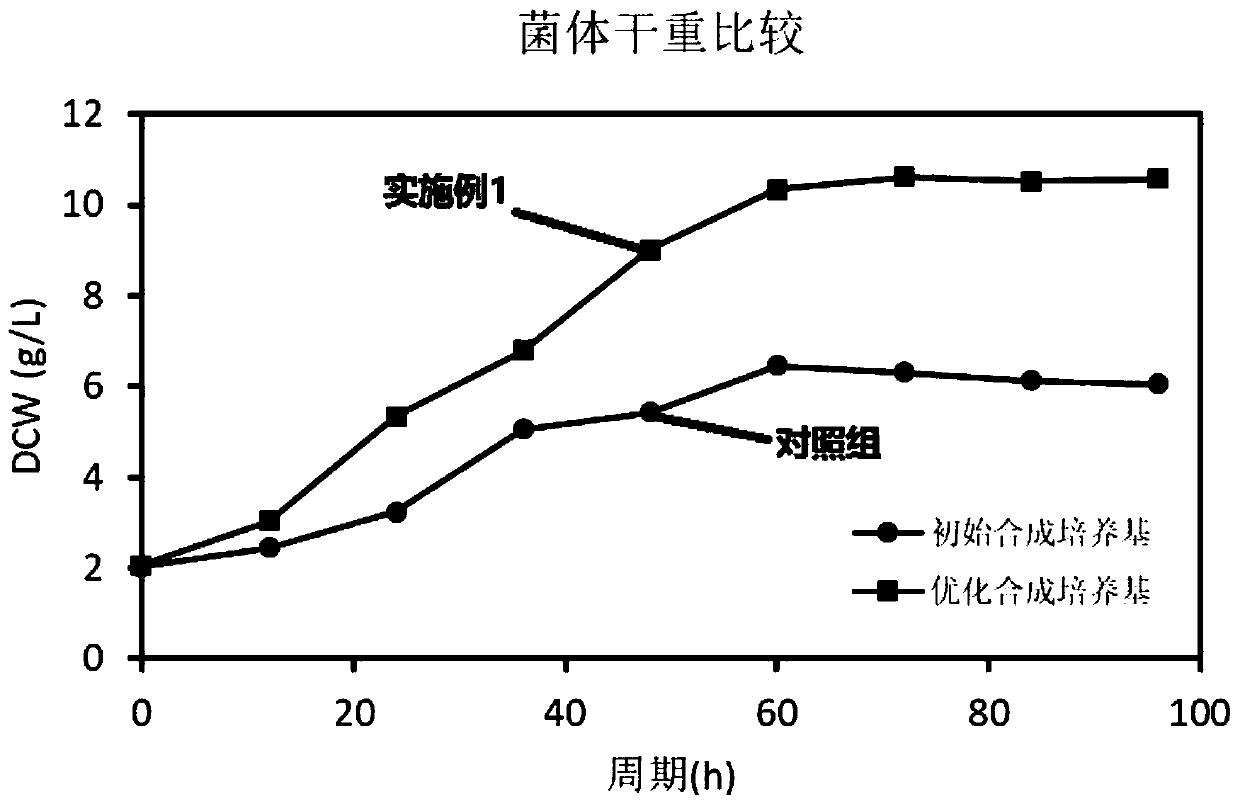
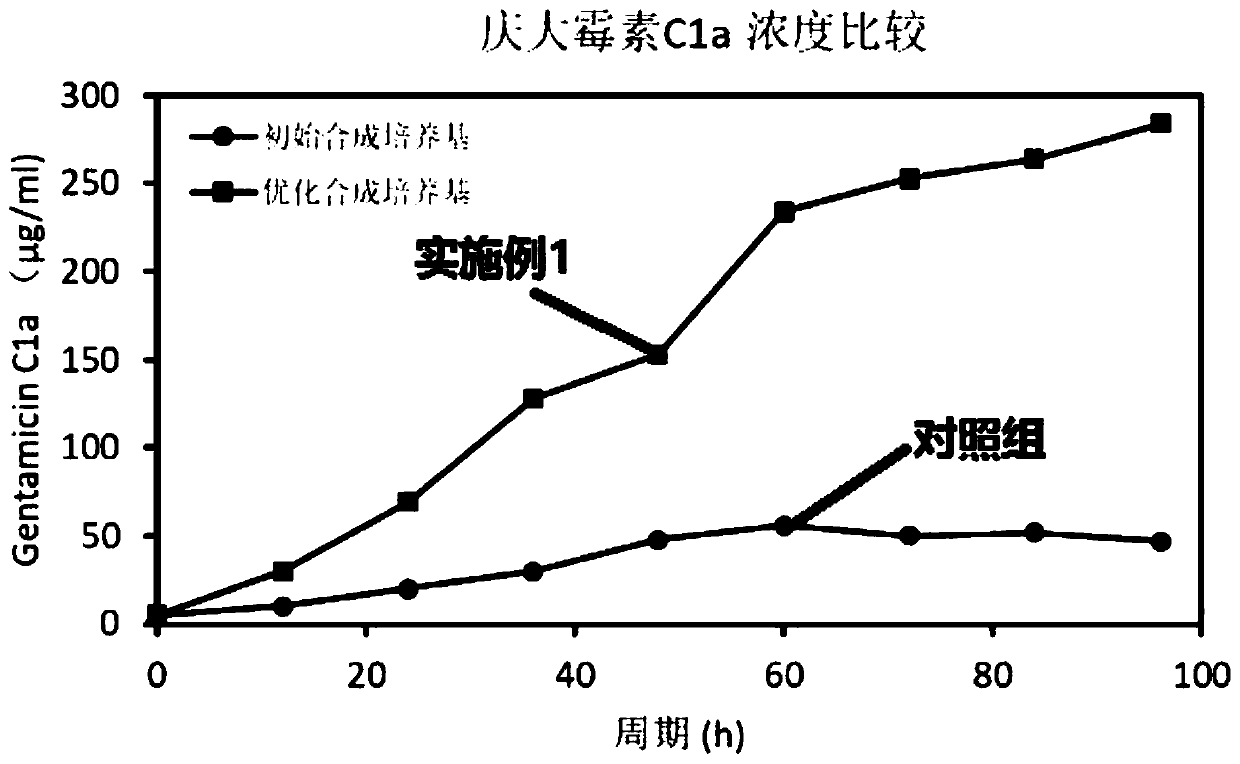
The invention discloses a synthetic medium as well as a preparation method and a use thereof. The synthetic medium is characterized by being prepared from the following components by mass concentration: 10-30g / L of glucose.H2O, 0.2-1.0g / L of xylose, 3-10g / L of ammonium sulfate, 2-5g / L of sodium nitrate, 0.2-0.8g / L of potassium chloride, 1.0-3.0g / L of potassium dihydrogen phosphate, 0.02-0.08g / L offerrous sulfate, 0.03-0.06g / L of magnesium sulfate, 0.005-0.02g / L of cobalt chloride, 2-10g / L of calcium carbonate, 0.01-0.05g / L of phenylalanine, 0.001-0.01g / L of vitamin VB12, 0.001-0.02g / L of vitamin VB3, 0. 001-0.03g / L of biotin, 0.01-0.1g / L of betaine and the balance of water. The novel culture medium disclosed by the invention is used for culturing micromonospra and has an important significance of mechanism study on synthesizing secondary metabolites with fermentation of the micromonospra and industrial production of gentamicin C1a.
Owner:EAST CHINA UNIV OF SCI & TECH
Preparation method of 1-N-ethyl gentamicin Cla
ActiveCN101928311BAvoid Alkylation Side ReactionsAvoid hydrolysisSugar derivativesSugar derivatives preparationAlkyl transferPotassium borohydride
The invention discloses a preparation method of 1-N-ethyl gentamicin Cla, which comprises the following steps of: reacting 3,2',6'-tri-N-acetyl gentamicin Cla with hexamethyldisilazane in a trichloromethane solvent under the catalysis of concentrated sulfuric acid to generate 3,2',6'-tri-N-acetyl-5,2'',4''-tri(trimethylsilyl) gentamicin Cla; carrying out N-alkylation reaction on the 3,2',6'-tri-N-acetyl-5,2'',4''-tri(trimethylsilyl) gentamicin Cla and acetaldehyde in a dichloromethane solvent at a temperature of 8-12 DEG C; carrying out reduction reaction with potassium borohydride; then hydrolyzing with an NaOH solution to obtain hydrolyzate of the 1-N-ethyl gentamicin Cla; and post-processing the hydrolyzate to obtain a finished product of the 1-N-ethyl gentamicin Cla. The method has the advantage of higher yield of the1-N-ethyl gentamicin Cla.
Owner:CHANGZHOU FANGYUAN PHARMA +1
A kind of preparation method of 1-n-ethyl gentamicin c1a
The invention relates to a preparation method of 1-N-ethyl gentamicin C1a. The method comprises the following steps: under the condition of 18-20 DEG C, slowly adding sodium borohydride into three flasks with trichloromethane and glacial acetic acid, dissolving 3,2',6'-tri-N-gentamicin C1a into trichloromethane, slowly adding the mixture into the flasks through a constant pressure dropping funnel, keeping the temperature to 40-50 DEG C, vigorously stirring for reaction 20 hours, cooling and adding a saturated sodium hydroxide aqueous solution to neutralize the system, diluting with ethyl alcohol, extracting with trichloromethane, performing vacuum concentration to obtain the oily matter, and adding a 1N sodium hydroxide aqueous solution for circulation reflux for 24 hours; diluting with salt-free water, performing column chromatography isolation, collecting effective constituents, concentrating and freeze-drying to obtain 1-N-ethyl gentamicin C1a.
Owner:WUXI JIYU SHANHE PHARM CO LTD +1
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com