Synthesizing method of 3-N-ethyl gentamicin C1a
A technology of ethyl gentamicin and diacetyl gentamicin, applied in the field of organic chemical synthesis, can solve problems such as difficulty in obtaining impurities, no standard product sales of 3-N-ethyl gentamicin C1a, etc. , to achieve the effect of low cost, short time and less environmental pollution
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
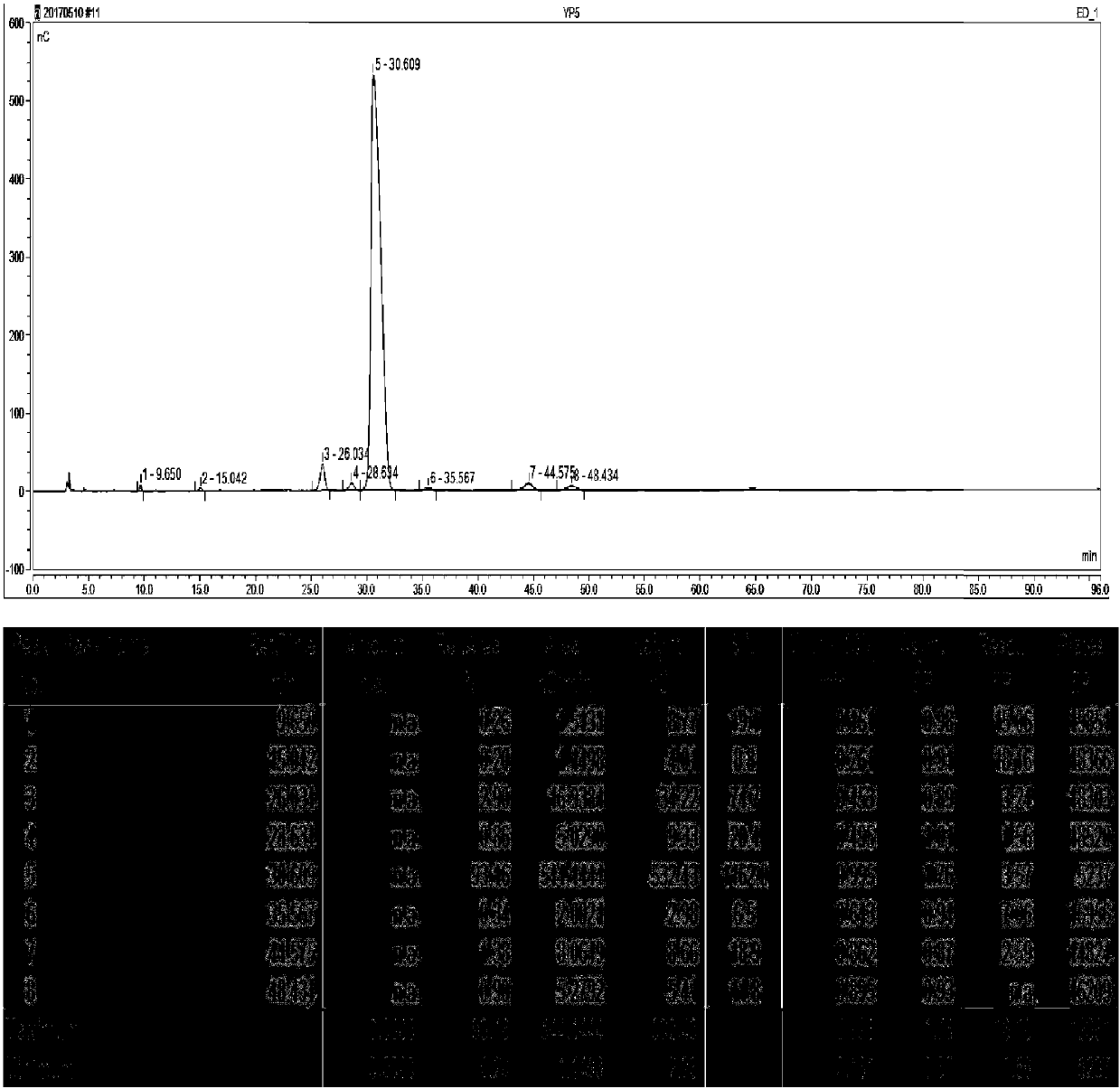
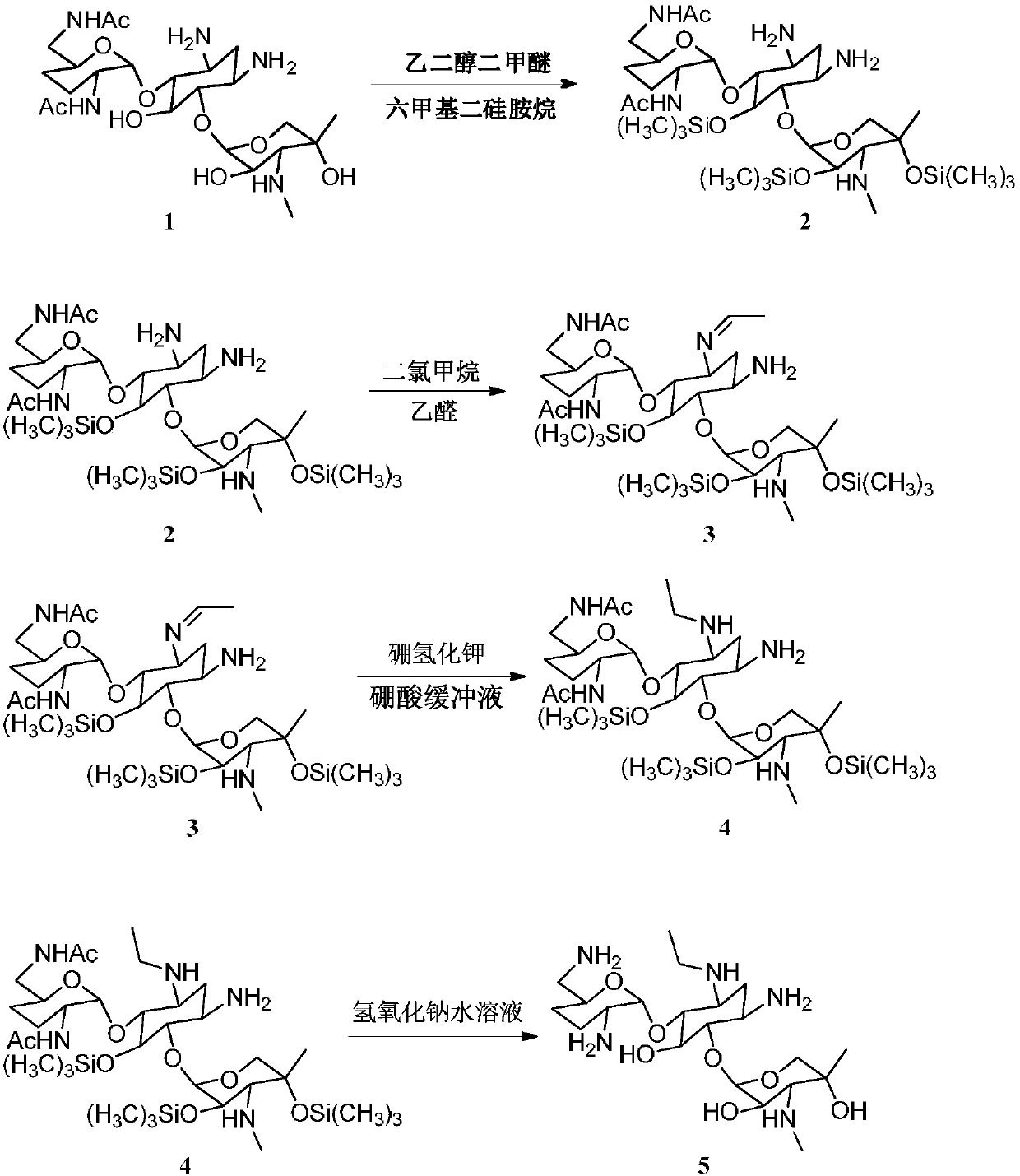
[0035] Embodiment 1, 3-N-ethyl gentamicin C 1a
[0036] a. Put 8 mL of ethylene glycol dimethyl ether, 5 mL of hexamethyldisilazane, and 0.02 mL of concentrated sulfuric acid into a 100 mL dry round-bottomed flask equipped with a reflux condenser with a drying tube. After stirring evenly, add 2" ,6″-N,N-Diacetylgentamycin C 1a 1.0g, heated to reflux, reacted for 2h. Change the distillation device to heat at normal pressure to steam out the solvent ethylene glycol dimethyl ether.
[0037] b. Cool down to 0-10°C, add 9mL of dichloromethane, then add dropwise 0.2mL of acetaldehyde at 0-10°C, and stir for 1h. Add 0.5 g of potassium borohydride, react for 0.5 h, add 3 mL of boric acid buffer solution (1.0 g of boric acid and 3.0 mL of deionized water are stirred to dissolve, adjust pH=10 with sodium hydroxide), and stir for 1.5 h.
[0038] c. Add 10% sodium hydroxide and stir for 0.5h, heat and distill under normal pressure until the liquid temperature is 100°C, and distill of...
Embodiment 2
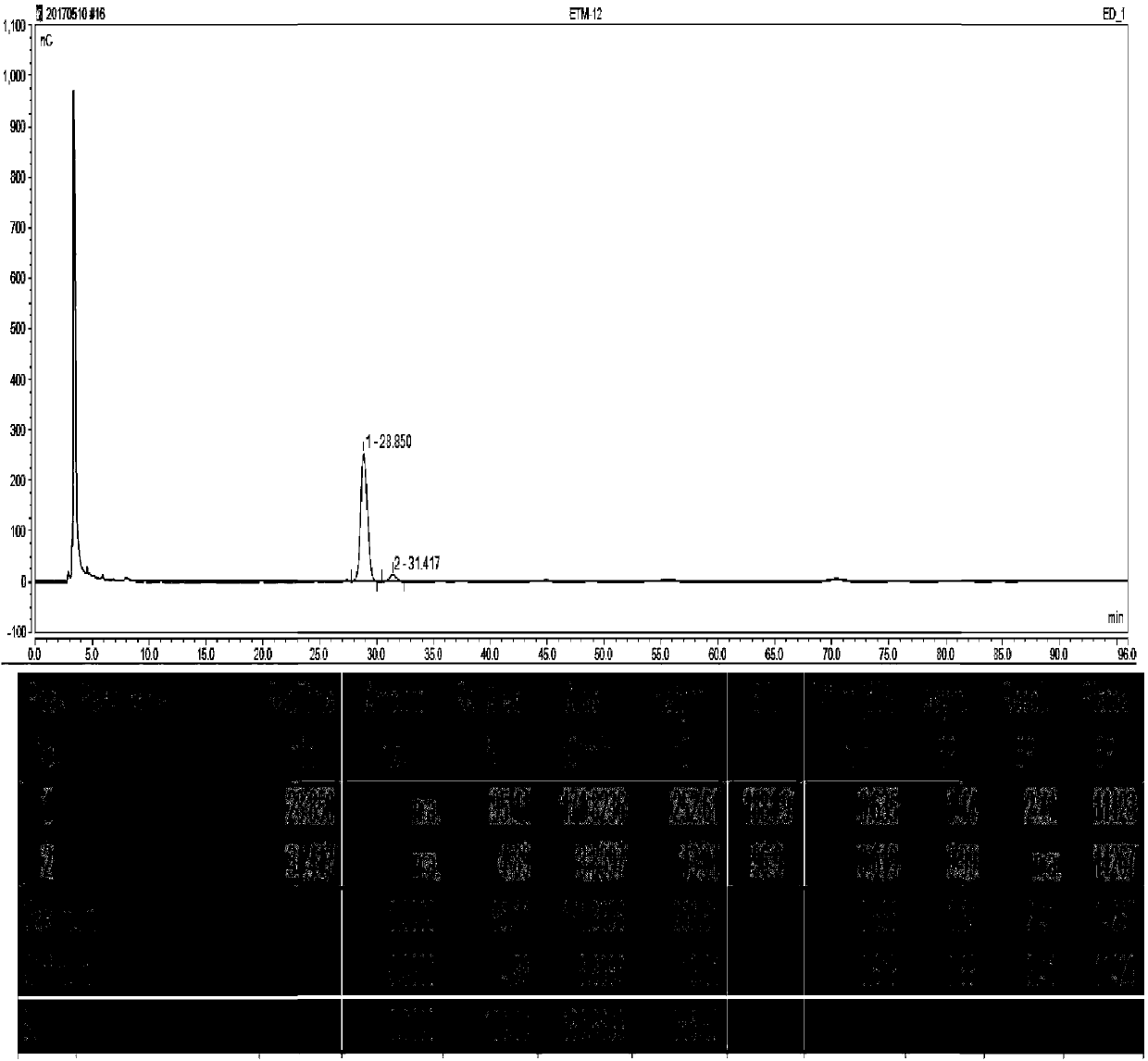
[0040] Embodiment 2, 3-N-ethyl gentamicin C 1a
[0041] a. Put 8 mL of ethylene glycol dimethyl ether, 5 mL of hexamethyldisilazane, and 0.02 mL of concentrated sulfuric acid into a 100 mL dry round-bottomed flask equipped with a reflux condenser with a drying tube. After stirring evenly, add 2" ,6″-N,N-Diacetylgentamycin C 1a 1.0g, heated to reflux, reacted for 2h. Change the distillation device to heat at normal pressure to steam out the solvent ethylene glycol dimethyl ether.
[0042] b. Cool down to 0-10°C, add 9mL of dichloromethane, then add dropwise 0.2mL of acetaldehyde at 0-10°C, and stir for 1h. Add 0.5 g of potassium borohydride, react for 0.5 h, add 3 mL of boric acid buffer solution (1.0 g of boric acid and 3 mL of deionized water are stirred to dissolve, adjust pH=10 with sodium hydroxide), and stir for 1.5 h.
[0043]c. Add 10% sodium hydroxide and stir for 0.5h, heat and distill under normal pressure until the liquid temperature is 100°C, and distill off p...
Embodiment 3
[0045] Embodiment 3, 3-N-ethyl gentamicin C 1a
[0046] a. Put 8 mL of ethylene glycol dimethyl ether, 5 mL of hexamethyldisilazane, and 0.02 mL of concentrated sulfuric acid into a 100 mL dry round-bottomed flask equipped with a reflux condenser with a drying tube. After stirring evenly, add 2" ,6″-N,N-Diacetylgentamycin C 1a 1.0g, heated to reflux, reacted for 2h. Change the distillation device to heat at normal pressure to steam out the solvent ethylene glycol dimethyl ether.
[0047] b. Cool down to 0-10°C, add 9mL of dichloromethane, then add dropwise 0.2mL of acetaldehyde at 0-10°C, and stir for 1h. Add 0.5 g of potassium borohydride, react for 0.5 h, add 3 mL of boric acid buffer solution (1.0 g of boric acid and 3 mL of deionized water are stirred to dissolve, adjust pH=10 with sodium hydroxide), and stir for 1.5 h.
[0048] c. Add 10% sodium hydroxide and stir for 0.5h, heat and distill under normal pressure until the liquid temperature is 100°C, and distill off ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com