Synthesis method of 6''-N-ethyl gentamicin C1a
A technology of ethyl gentamicin and triacetyl gentamicin, which is applied in the field of synthesis of intermediate products of pharmaceutical compounds, can solve problems such as difficulty in obtaining, and achieve less side reactions, high synthesis yield, and easy purification and separation Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
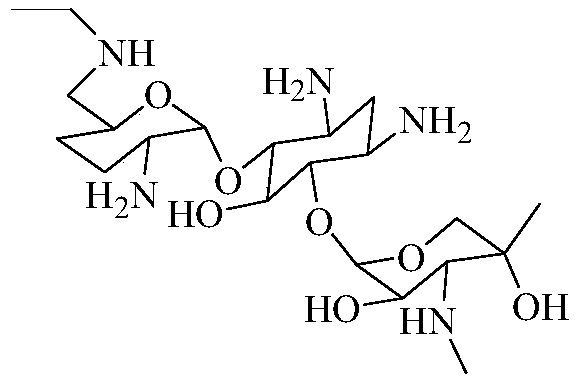
[0055] Embodiment 1, 6 "-N-ethyl gentamycin C1a
[0056] 1. Dissolve gentamicin C1a (5g) in tetrahydrofuran / triethylamine / water (25mL / 25mL / 25mL), and cool to -5~0°C. Add BOC-ON (2-tert-butoxycarbonyliminophenylacetonitrile) (1.0eq) and stir for 1-2 hours. Add 50mL of water and 50mL of ethyl acetate, separate the layers, take the aqueous phase and concentrate.
[0057] 2. Dissolve the concentrate in 50mL of methanol, add 10mL of triethylamine, cool to 0-10°C, add acetic anhydride (4-5eq), and stir for 0.5-1 hour. Concentrate to remove solvent.
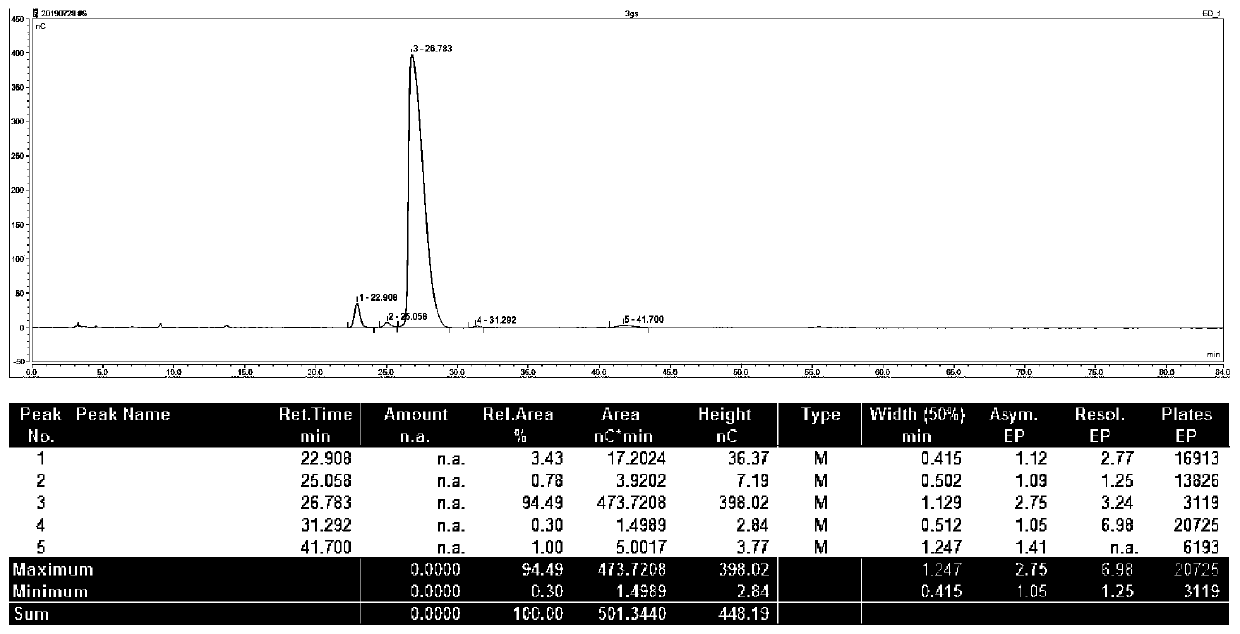
[0058] 3. Add 50-75 mL of 3 mol / L hydrochloric acid to the concentrated solution, and stir at room temperature for 1-3 hours. Adjust the pH to 8~9 with sodium hydroxide solution, concentrate, and purify and separate 1,3,2"-N,N,N-triacetylgentamycin C1a with silica gel column. Among them, the specific packing type and size of silica gel column , the elution method and other conditions are as follows:
[0059] Silica gel column model...
Embodiment 2
[0065] Embodiment 2, 6 "-N-ethyl gentamycin C1a
[0066] 1. Dissolve gentamicin C1a (5g) in tetrahydrofuran / triethylamine / water (25mL / 25mL / 25mL), and cool to -5~0°C. Add BOC-ON (2-tert-butoxycarbonyliminophenylacetonitrile) (1.0eq) and stir for 1-2 hours. Add 50mL of water and 50mL of ethyl acetate, separate the layers, take the aqueous phase and concentrate.
[0067] 2. Dissolve the concentrate in 50mL of methanol, add 10mL of triethylamine, cool to 0-10°C, add acetic anhydride (4-5eq), and stir for 0.5-1 hour. Concentrate to remove solvent.
[0068] 3. Add 50-75 mL of 3 mol / L hydrochloric acid to the concentrated solution, and stir at room temperature for 1-3 hours. Adjust the pH to 8~9 with sodium hydroxide solution, concentrate, and purify and separate 1,3,2”-N,N,N-triacetylgentamycin C1a with silica gel column. Among them, the specific packing type and size of the silica gel column , the elution method and other conditions are as follows:
[0069] Silica gel column m...
Embodiment 3
[0078] Embodiment 3, 6 "-N-ethyl gentamycin C1a
[0079] 1. Dissolve gentamicin C1a (5g) in tetrahydrofuran / triethylamine / water (25mL / 25mL / 25mL), and cool to -5~0°C. Add BOC-ON (2-tert-butoxycarbonyliminophenylacetonitrile) (1.0eq) and stir for 1-2 hours. Add 50mL of water and 50mL of ethyl acetate, separate the layers, take the aqueous phase and concentrate.
[0080] 2. Dissolve the concentrate in 50mL of methanol, add 10mL of triethylamine, cool to 0-10°C, add acetic anhydride (4-5eq), and stir for 0.5-1 hour. Concentrate to remove solvent.
[0081] 3. Add 50-75 mL of 3 mol / L hydrochloric acid to the concentrated solution, and stir at room temperature for 1-3 hours. Adjust the pH to 8-9 with sodium hydroxide solution, concentrate, and purify with silica gel column to separate 1,3,2"-N,N,N-triacetylgentamycin C1a.
[0082] Among them, the specific filler type, size, and elution method of the silica gel column are as follows:
[0083] Silica gel column model: ZCX Ⅱ, reage...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com