Preparation method of 1-N-ethyl gentamicin C1a sulfate
A technology of ethyl gentamicin and base gentamycin, which is applied in the field of preparation of medicinal raw materials, can solve problems such as high requirements and complicated operation, and achieve the effect of increasing yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1)
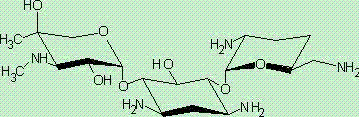
[0036] ①Add 1000mL of methanol solvent and 90g of gentamicin C 1a (0.2mol) and 110g of zinc acetate, stirred to fully dissolve, gentamicin C 1a (I) Coordination reaction with zinc acetate to obtain gentamicin C 1a -Zn complexes.
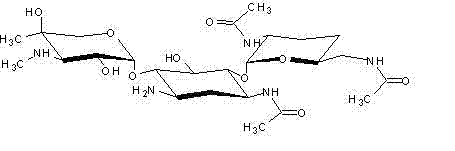
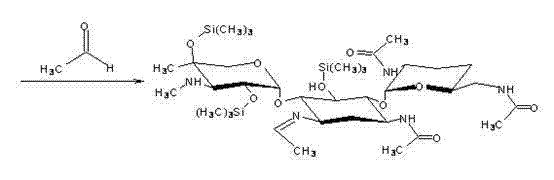
[0037] ②Reduce the temperature of the system in step ① to 0°C to 10°C, add dropwise a mixture of 85mL acetic anhydride (0.9mol), 350mL triethylamine and 150mL tetrahydrofuran to the system in step ① under stirring, and generate 3,2',6'-tri-N-acetylgentamycin C 1a For the acylation reaction of the Zn complex, continue stirring and insulated reaction for 1 h after the dropwise completion. Then add 300mL of purified water to the reacted material, then place it in a rotary evaporator and distill under vacuum and reduced pressure at a temperature of 60°C to obtain 3,2',6'-tri-N-acetyl genta Mycin C 1a -300 mL of the concentrated solution of the Zn complex, and then cooled to 30°C.
[0038] ③Post-treatment the concentrated solution obtained in step ②...
Embodiment 2)
[0046] The rest of this embodiment is the same as Example 1, except that step 3.: dilute the concentrated solution obtained in step 2. with water to 2000mL, and then use the DL8040 nanofiltration membrane produced by DE Company of the United States to carry out nanofiltration treatment on the diluted solution until The concentration of zinc ions in the diluent is ≤10ug / mL, and then the nanofiltrate is placed in a rotary evaporator and vacuum-reduced at a temperature of 60°C to obtain a 50mL concentrated solution, and finally the concentrated solution is spray-dried to obtain 110g of powdered 3,2 ',6'-tri-N-acetylgentamycin C 1a , the yield reached 94.9%, and the purity reached 92%.
Embodiment 3~ Embodiment 6)
[0048] The preparation method of each embodiment is basically the same as that of Example 1, and the difference is that the acetic anhydride used in step 2. and gentamicin C 1a The molar ratio of different, the influence of acetic anhydride dosage on the reaction yield is shown in Table 1.
[0049] Table 1
[0050] Acetic anhydride and gentamicin C 1a molar ratio yield Example 1 4.5∶1 93.9% Example 3 5∶1 93.6% Example 4 4∶1 90.2% Example 5 3.5∶1 87.9% Example 6 3∶1 85.4%
[0051] As can be seen from Table 1, increasing the amount of acetic anhydride increases the reaction yield, and when the molar ratio reaches 4.5:1, the yield decreases instead when the amount of acetic anhydride is increased. Therefore, acetic anhydride and Qing Damycin C 1a The optimum molar ratio is 4.5:1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com