Etimicin sulfate preparation method
A technology of etimicin sulfate and sodium carbonate, applied in the field of medicine, can solve the problems of poor selectivity of protective groups, many impurities in intermediates, and high industrialization cost, and achieve the effects of short reaction time, simple conditions and easy operation.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
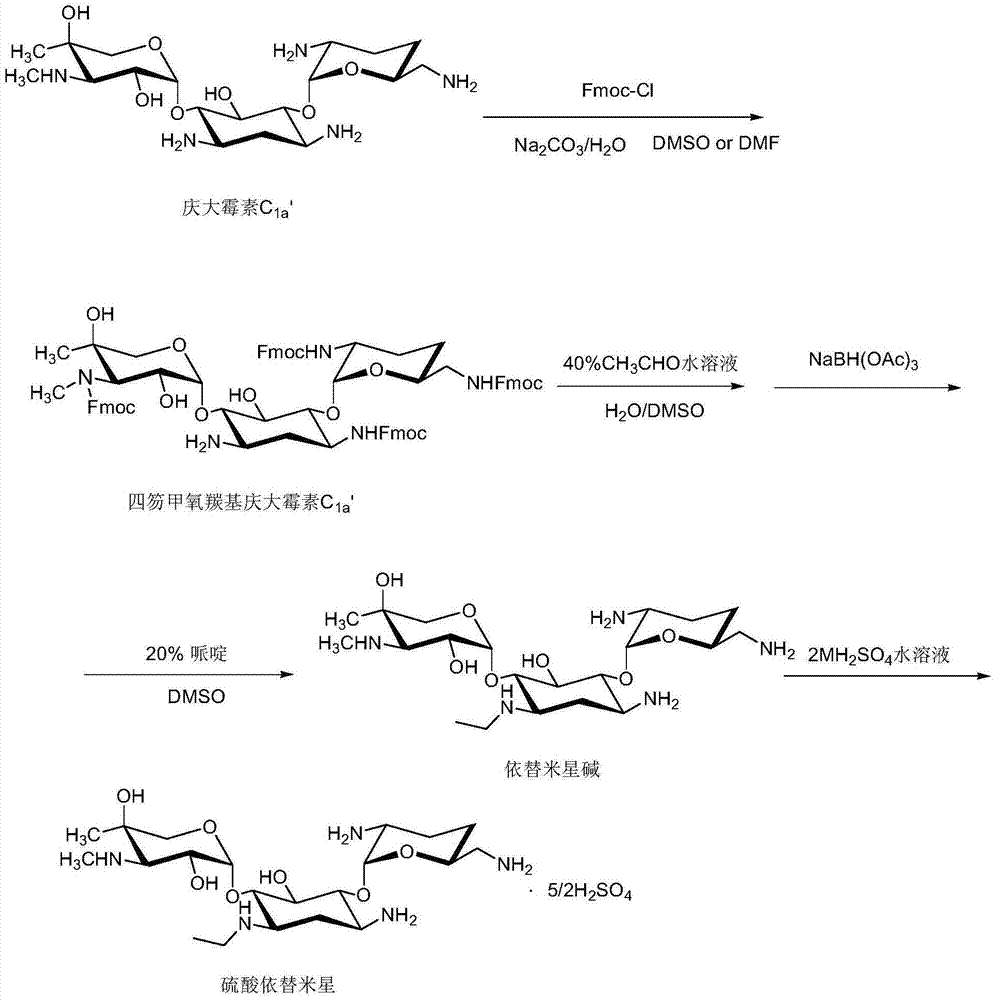
[0026] 25g of gentamicin C with a purity of not less than 98% 1a Dissolve in 500ml of DMSO, stir to dissolve, add 300ml of 10% sodium carbonate solution, cool down to 0°C, then add 65.2g / 200ml of Fmoc-Cl / DMSO solution dropwise, dropwise in 20-40 minutes, drop After the addition, react at 0-3°C for 2 hours, and then react at room temperature for 2 hours; inject the reaction solution into HPD-950 macroporous adsorption resin for adsorption, first rinse with water, and then analyze with 30% ethanol solution to obtain the analysis solution of the desired product, thin film Evaporator concentrated to a small volume and then evaporated to dryness under reduced pressure to obtain a yellow oil; EI (m / z): 1338 (M+1);
[0027] Dissolve the above yellow oil in 150ml of water and 100ml of DMSO mixture, cool down to 0-5°C, adjust the pH value to 2.5-3.0 with hydrochloric acid, add 12.3ml of 40% acetaldehyde aqueous solution after dissolving, and control the temperature at 0-5°C. React at ...
Embodiment 2
[0031] 25g of gentamicin C with a purity of not less than 98% 1a Dissolve in 500ml of DMSO, stir to dissolve, add 350ml of 10% sodium carbonate solution, lower the temperature to 0°C, and then add 68.1g / 200ml of Fmoc-Cl / DMSO solution dropwise to it, the dropwise addition is completed in 20-40 minutes, dropwise After completion, react at 0-3°C for 2 hours, and then react at room temperature for 2 hours; inject the reaction solution into HPD-950 macroporous adsorption resin for adsorption, rinse with water first, and then analyze with 30% ethanol solution to obtain the analysis solution of the desired product, and evaporate the film Evaporate to dryness under reduced pressure to obtain a yellow oil after being concentrated to a small volume; EI (m / z): 1338 (M+1);
[0032] Dissolve the above yellow oil in 160ml of water and 100ml of DMSO mixture, cool down to 0-5°C, adjust the pH value to 2.5-3.0 with hydrochloric acid, add 12.3ml of 40% acetaldehyde / water solution after dissolvi...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com